Volume 10, Issue 2 (June 2025)
J Environ Health Sustain Dev 2025, 10(2): 2632-2642 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hatami B, Rezaie A, Malekbala M. Mineralization of Azo Dye from Aqueous Solution via Electro-Activated Persulfate: A Case Study on Direct Red 89. J Environ Health Sustain Dev 2025; 10 (2) :2632-2642
URL: http://jehsd.ssu.ac.ir/article-1-947-en.html
URL: http://jehsd.ssu.ac.ir/article-1-947-en.html
Environmental Sciences and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 686 kb]
(184 Downloads)
| Abstract (HTML) (686 Views)
.JPG)
.JPG)
.JPG)
Figure 3: The effect of current density on DR 89 removal efficiency by EC/PS process (PH: 4, DR 89 concentration: 80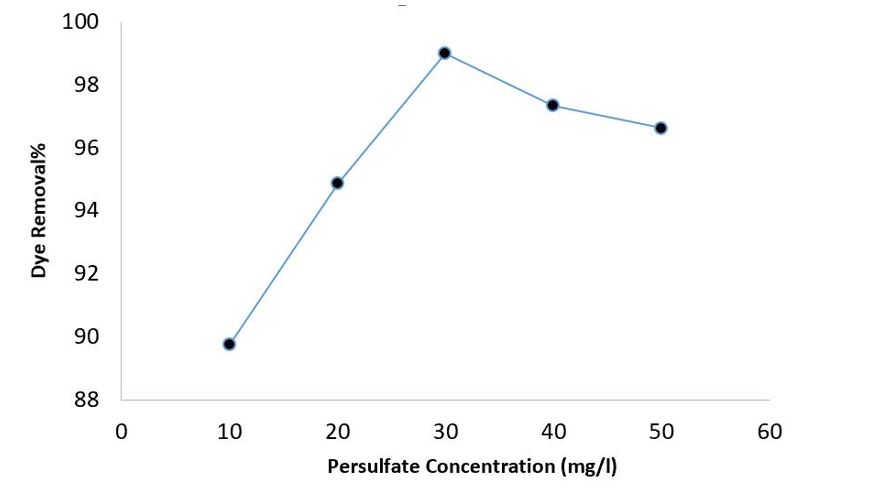
Full-Text: (169 Views)
Behnam Hatami 1, Arezo Rezaie 1*, Maryam Malekbala 2
1 Environmental Sciences and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Department of environmental health engineering, school of public health, student research committee, Hamadan university of medical sciences, Hamadan, Iran.
2 Department of environmental health engineering, school of public health, student research committee, Hamadan university of medical sciences, Hamadan, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: In recent years, azo dyes, which are widely used in various industries, have attracted attention because of their high production volume, toxicity, and environmental persistence. Advanced oxidation processes (AOPs) have emerged as promising alternatives for the degradation of pollutants by generating reactive radicals. This study investigated the degradation of Direct Red 89 (DR 89) using the electrochemical/persulfate (EC/PS) process. Materials and Methods: A controlled laboratory experiment was conducted utilizing a 1000 mL electrolytic reactor, which was equipped with aluminum and iron electrodes serving as the anode and cathode, respectively. The reactor contained 500 mL of solution, which was continuously stirred using a magnetic stirrer. Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were used to adjust the pH, while sodium chloride (NaCl) served as the supporting electrolyte. The concentration of DR 89 in the samples was measured using a UV-visible spectrophotometer. Results: The investigation of operational parameters, including pH, reaction time, persulfate concentration, current density, initial dye concentration, and electrolyte concentration, indicated that a maximum removal efficiency of 99.43% was achieved under the optimal conditions: pH 4.0, reaction time of 25 min, current density of 1 mA/cm², electrolyte concentration of 250 mg/L, and persulfate concentration of 30 mg/L, for an aqueous solution containing 80 mg/L of DR89 dye. Conclusion: Compared with other advanced oxidation processes, this approach is more environmentally friendly, with high efficiency, and less pollutant production. Therefore, it can be widely used to treat industrial wastewater containing persistent pollutants. |
|
Article History: Received: 23 February 2025 Accepted: 20 May 2025 |
||
*Corresponding Author: Arezo Rezaie Email: arezorezaie373@gmail.com Tel: +98 913 2204694 |
||
Keywords: Azo Dyes, Direct Red 89, Electrochemical Persulfate Activation. |
Citation: Hatami B, Rezaie A, Malekbala M. Mineralization of Azo Dye from Aqueous Solution via Electro-Activated Persulfate: A Case Study on Direct Red 89. J Environ Health Sustain Dev. 2025; 10(2): 2632-42.
Introduction
In recent years, azo dyes have constituted the largest production volume among chemical dyes, and their significance is expected to increase in various industries, particularly in the textile sector1. Annually, approximately 280,000 tons of textile dyes are discharged into industrial wastewater worldwide, with Azo dyes constituting approximately 70% of this total.2. These compounds contain azo groups (-N=N-) attached to aromatic rings, along with other groups such as -OH, -SO3-, and -NO2 3. Most of these compounds are non-degradable, toxic, mutagenic, and inherently carcinogenic due to their aromatic molecular structure.4.
Various chemical and physical techniques, including chemical precipitation for pollutant separation, electrocoagulation, and activated carbon adsorption, have been employed to remove dyes from diverse wastewater sources.5, 6. However, these technologies are less frequently applied to challenges such as high sludge production, high sensitivity to variations in flow rate and organic load, extended retention time, and low removal efficiency. 2.
AOPs have emerged as alternatives to conventional technologies. In an oxidation reaction, one or more electrons are transferred from one chemical to another, which is known as the oxidant. The removal of contaminants in advanced oxidation processes is based on the production of hydroxyl radicals, which are powerful oxidizing agents that convert many organic compounds into inorganic substances. This radical is characterized by its instability and high reactivity and is generated in situ through either chemical or photochemical reactions.7, 8.
Recently, an alternative form of AOPs has been explored, which is based on the generation of sulfate radicals. These radicals are characterized by a longer half-life than hydroxyl radicals.9. Persulfate (PS, S2O82-, E0 = 2.01 V) is commonly used as a strong oxidizer in sulfate radical-based AOPs because of its high stability and reasonable cost 10.
Persulfate can be activated through various energy sources, including thermal energy11, ultraviolet radiation12, microwave radiation13, Ultrasound14. or electron transfer metal transfer15, leading to the generation of sulfate radicals (Equation 1-4) 16, 17.
S2O82- + heat 2SO4- (1)
S2O82- + hv 2SO4- (2)
S2O82-+Mn2+ SO4-+M (n+1) + SO42- (3)
S2O82- + Ultrasound 2SO4- (4)
Divalent iron (Fe²⁺) is a crucial catalyst for persulfate activation, facilitating the generation of sulfate radicals (SO₄•⁻). Its presence is instrumental in enhancing the degradation of organic pollutants in the environment. Once formed in solution, SO₄•⁻ can rapidly react with a wide range of organic pollutants through strong oxidation reactions 15, 18. Consequently, the degradation of contaminants such as toluene19, Metformin20, and levofloxacin21 in aqueous solutions has been examined. To the best of our knowledge, this study is the first to report the removal of DR 89 using the EC/PS method. This study examined key operational parameters, including reaction time, current density, persulfate concentration, pH, electrolyte concentration, and initial dye concentration, to assess the removal efficiency of DR 89 in the EC/PS process.
Materials and Methods
Material
All chemicals used in the experiments were of analytical grade. Direct Red 89 (CAS No. 12217-67-3) and sodium persulfate (CAS No. 7772-98-7) were purchased from Merck. (Germany (. Table 1 presents the chemical structure and main characteristics of DR 89. Sulfuric acid (H2SO4) and sodium hydroxide (NaOH) used for adjusting the pH were provided by Sigma-Aldrich.
In recent years, azo dyes have constituted the largest production volume among chemical dyes, and their significance is expected to increase in various industries, particularly in the textile sector1. Annually, approximately 280,000 tons of textile dyes are discharged into industrial wastewater worldwide, with Azo dyes constituting approximately 70% of this total.2. These compounds contain azo groups (-N=N-) attached to aromatic rings, along with other groups such as -OH, -SO3-, and -NO2 3. Most of these compounds are non-degradable, toxic, mutagenic, and inherently carcinogenic due to their aromatic molecular structure.4.
Various chemical and physical techniques, including chemical precipitation for pollutant separation, electrocoagulation, and activated carbon adsorption, have been employed to remove dyes from diverse wastewater sources.5, 6. However, these technologies are less frequently applied to challenges such as high sludge production, high sensitivity to variations in flow rate and organic load, extended retention time, and low removal efficiency. 2.
AOPs have emerged as alternatives to conventional technologies. In an oxidation reaction, one or more electrons are transferred from one chemical to another, which is known as the oxidant. The removal of contaminants in advanced oxidation processes is based on the production of hydroxyl radicals, which are powerful oxidizing agents that convert many organic compounds into inorganic substances. This radical is characterized by its instability and high reactivity and is generated in situ through either chemical or photochemical reactions.7, 8.
Recently, an alternative form of AOPs has been explored, which is based on the generation of sulfate radicals. These radicals are characterized by a longer half-life than hydroxyl radicals.9. Persulfate (PS, S2O82-, E0 = 2.01 V) is commonly used as a strong oxidizer in sulfate radical-based AOPs because of its high stability and reasonable cost 10.
Persulfate can be activated through various energy sources, including thermal energy11, ultraviolet radiation12, microwave radiation13, Ultrasound14. or electron transfer metal transfer15, leading to the generation of sulfate radicals (Equation 1-4) 16, 17.
Divalent iron (Fe²⁺) is a crucial catalyst for persulfate activation, facilitating the generation of sulfate radicals (SO₄•⁻). Its presence is instrumental in enhancing the degradation of organic pollutants in the environment. Once formed in solution, SO₄•⁻ can rapidly react with a wide range of organic pollutants through strong oxidation reactions 15, 18. Consequently, the degradation of contaminants such as toluene19, Metformin20, and levofloxacin21 in aqueous solutions has been examined. To the best of our knowledge, this study is the first to report the removal of DR 89 using the EC/PS method. This study examined key operational parameters, including reaction time, current density, persulfate concentration, pH, electrolyte concentration, and initial dye concentration, to assess the removal efficiency of DR 89 in the EC/PS process.
Materials and Methods
Material
All chemicals used in the experiments were of analytical grade. Direct Red 89 (CAS No. 12217-67-3) and sodium persulfate (CAS No. 7772-98-7) were purchased from Merck. (Germany (. Table 1 presents the chemical structure and main characteristics of DR 89. Sulfuric acid (H2SO4) and sodium hydroxide (NaOH) used for adjusting the pH were provided by Sigma-Aldrich.
Table 1: Chemical structure and main characteristics of DR89.
| Name | Direct Red 89 |
| molecular structure | |
| Chemical formula Molecular weight Cass Number |
C44H32N10Na4O16S4 1177.0 12217-67-3 |
Pilot setup
The experiments were conducted in an electrolytic reactor with a working volume of 1000 ml, containing 500 ml of the solution. The cathode and anode electrodes, composed of aluminum and iron with a purity of 99.95% by weight, respectively, measured 16 cm × 6 cm × 2 mm and were submerged in the electrolyte. The effective surface area of each electrode was 147.2 cm2, and the spacing between the electrodes was maintained at 2 cm, with the electrodes arranged in parallel. Electrolysis was performed using a direct current (DC) power supply (DAZHENG PS-305D).
A magnetic stirrer was used to agitate the reactor. The initial pH was adjusted using NaOH (1N) and HCl (1N). Sodium chloride (NaCl) was added as a supporting electrolyte to the aqueous dye solution. The desired amount of persulfate was then added to the sample solution. Subsequently, the samples were collected from the solution and centrifuged at 3500 rpm for 5 min22. Table 2 summarizes the experimental conditions, including the key operational parameters and investigated ranges.
The experiments were conducted in an electrolytic reactor with a working volume of 1000 ml, containing 500 ml of the solution. The cathode and anode electrodes, composed of aluminum and iron with a purity of 99.95% by weight, respectively, measured 16 cm × 6 cm × 2 mm and were submerged in the electrolyte. The effective surface area of each electrode was 147.2 cm2, and the spacing between the electrodes was maintained at 2 cm, with the electrodes arranged in parallel. Electrolysis was performed using a direct current (DC) power supply (DAZHENG PS-305D).
A magnetic stirrer was used to agitate the reactor. The initial pH was adjusted using NaOH (1N) and HCl (1N). Sodium chloride (NaCl) was added as a supporting electrolyte to the aqueous dye solution. The desired amount of persulfate was then added to the sample solution. Subsequently, the samples were collected from the solution and centrifuged at 3500 rpm for 5 min22. Table 2 summarizes the experimental conditions, including the key operational parameters and investigated ranges.
Table 2: Experimental conditions employed in DR89 degradation by PS-EC process
| Studied parameter |
Experiment condition | |||||
| pH | Time (min) |
Current density (mA/cm2) |
PS dose (mg/L) |
NaCl (mg/L) |
dye conc. (mg/L) |
|
| Solution pH | 3-9 | 20 | 0.67 | 25 | 250 | 80 |
| Reaction time | 4 | 5-90 | 0.67 | 25 | 250 | 80 |
| Current density | 4 | 25 | 0.67-2.37 | 25 | 250 | 80 |
| PS dose | 4 | 25 | 1 | 10-50 | 250 | 80 |
| Electrolyte dose | 4 | 25 | 1 | 30 | 60-450 | 80 |
| Initial color concentration |
4 | 35 | 1 | 30 | 250 40-120 | |
Analytical methods
The amount of DR-98 adsorbed in the samples was measured at.JPG) nm using UV– visible spectrophotometer (DR5000 © Hach Company)23. A calibration curve was drawn using the prepared paint solutions ranging from 0.125 to 25 mg/L, and the linear correlation coefficient was calculated (R2= 0.993). The removal efficiency (RE) of DR 89 was calculated using Equation (4).
nm using UV– visible spectrophotometer (DR5000 © Hach Company)23. A calibration curve was drawn using the prepared paint solutions ranging from 0.125 to 25 mg/L, and the linear correlation coefficient was calculated (R2= 0.993). The removal efficiency (RE) of DR 89 was calculated using Equation (4).
RE =C C C
where C0 and C are the concentrations of the sample at time 0 and the residual concentration, respectively.
Results
Effect of solution pH
This study examined the influence of pH levels ranging from 3.0 to 9.0 on the removal of DR 89. Figure 1 illustrates the removal efficiency of DR 89 as a function of the pH. As shown in Figure 1, the highest removal efficiency of DR 89 (98.87%) was observed at pH 4.0
The amount of DR-98 adsorbed in the samples was measured at
RE =
where C0 and C are the concentrations of the sample at time 0 and the residual concentration, respectively.
Results
Effect of solution pH
This study examined the influence of pH levels ranging from 3.0 to 9.0 on the removal of DR 89. Figure 1 illustrates the removal efficiency of DR 89 as a function of the pH. As shown in Figure 1, the highest removal efficiency of DR 89 (98.87%) was observed at pH 4.0
.JPG)
Figure 1: Effect of solution pH on the removal of DR 89 by EC/PS process (DR 89 concentration: 80 mg/L, PS concentration: 25 mg/L, electrolyte concentration: 250 mg/L, current density: 0.67 mA/cm2, and reaction time: 20 min)
Effect of reaction time
The effects of different reaction times (5-90 min) on the removal efficiency of DR 89 were investigated. As shown in Figure 2, the removal efficiency of DR89 increased to 88.38% within the first 5 min and reached 98.24% after 25 min in the EC/PS system. As the reaction time increased beyond 25 min, the removal efficiency decreased, ultimately declining to 94%.
The effects of different reaction times (5-90 min) on the removal efficiency of DR 89 were investigated. As shown in Figure 2, the removal efficiency of DR89 increased to 88.38% within the first 5 min and reached 98.24% after 25 min in the EC/PS system. As the reaction time increased beyond 25 min, the removal efficiency decreased, ultimately declining to 94%.
.JPG)
Figure 2: Effect of reaction time on DR 89 removal efficiency by EC/PS process (pH: 4, DR89 concentration: 80 mg/L, PS concentration: 25 mg/L, electrolyte concentration: 250mg/L, current density: 0.67 mA/cm2)
Effect of Current density
A current density range of 0.67-2.37 mA/cm2 was employed to investigate its effect on the removal efficiency of DR 89. As shown in Figure 3, increasing the current density from 0.67 to 1 mA/cm2 led to an increase in the removal efficiency from 97.67% to 98.04%, respectively. These results indicate that the removal of DR 89 increased with increasing current density.
A current density range of 0.67-2.37 mA/cm2 was employed to investigate its effect on the removal efficiency of DR 89. As shown in Figure 3, increasing the current density from 0.67 to 1 mA/cm2 led to an increase in the removal efficiency from 97.67% to 98.04%, respectively. These results indicate that the removal of DR 89 increased with increasing current density.
.JPG)
Figure 3: The effect of current density on DR 89 removal efficiency by EC/PS process (PH: 4, DR 89 concentration: 80
Effect of Persulfate concentration
Persulfate is integral to the EC/PS system because it is the primary source of SO₄•⁻. In this study, experiments were conducted at different PS concentrations ranging from 10 to 50 mg/l to evaluate its effect. Figure 4 depicts the effect of persulfate concentration on the oxidative degradation of DR 89. As shown in Figure 4, the removal efficiency of DR 89 increased from 89.76% to 98.98% as the PS concentration increased from 10 to 30 mg/L. As illustrated in Figure 4, when the persulfate concentration increased from 30 mg/L to 50 mg/L, the removal efficiency decreased from 98.98% to 96.63%.
Persulfate is integral to the EC/PS system because it is the primary source of SO₄•⁻. In this study, experiments were conducted at different PS concentrations ranging from 10 to 50 mg/l to evaluate its effect. Figure 4 depicts the effect of persulfate concentration on the oxidative degradation of DR 89. As shown in Figure 4, the removal efficiency of DR 89 increased from 89.76% to 98.98% as the PS concentration increased from 10 to 30 mg/L. As illustrated in Figure 4, when the persulfate concentration increased from 30 mg/L to 50 mg/L, the removal efficiency decreased from 98.98% to 96.63%.

Figure 4: The effect of PS dose on DR 89 removal efficiency by EC-PS process (pH: 4, DR89 concentration: 80mg/L, electrolyte concentration: 250 mg/L, current density: 1 mA/cm2 and reaction time: 25 min)
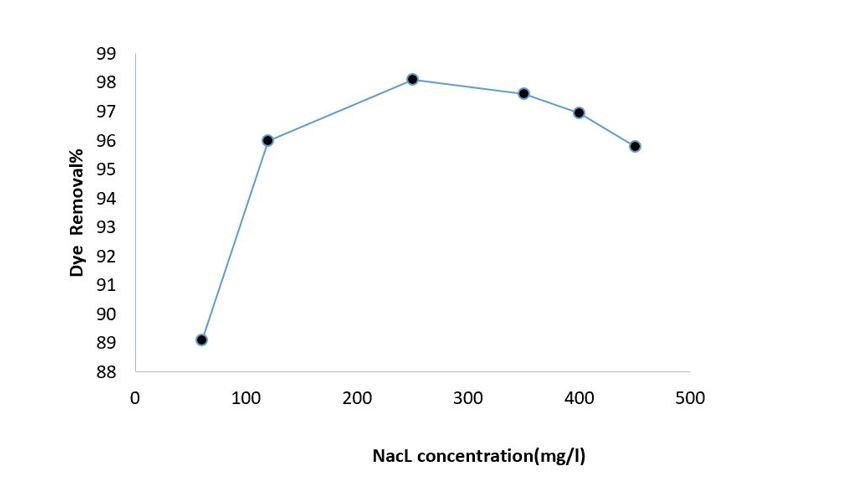
Effect of electrolyte concentration
To evaluate the effect of electrolyte concentration on the removal efficiency of DR 89 using the EC-PS process, various electrolyte concentrations ranging from 60 mg/L to 450 mg/L were investigated ) Figure.5). As shown in figure 5, increasing the electrolyte concentration from 60 mg/L to 250 mg/L led to an increase in the removal efficiency from 89.1% to 98.08%. However, at concentrations higher than 250 mg/L, the removal efficiency began to decrease, reaching 95.78% at an electrolyte concentration of 450 mg/L.
To evaluate the effect of electrolyte concentration on the removal efficiency of DR 89 using the EC-PS process, various electrolyte concentrations ranging from 60 mg/L to 450 mg/L were investigated ) Figure.5). As shown in figure 5, increasing the electrolyte concentration from 60 mg/L to 250 mg/L led to an increase in the removal efficiency from 89.1% to 98.08%. However, at concentrations higher than 250 mg/L, the removal efficiency began to decrease, reaching 95.78% at an electrolyte concentration of 450 mg/L.

Figure 5: Effect of electrolyte concentration on DR 89 removal efficiency by EC-PS process (PH: 4, DR 89 concentration: 80mg/L, PS concentration: 25mg/L, current density: 1 mA/cm2 and reaction time: 25 min)
In the electrolytic process, electrical conductivity is considered one of the most influential parameters affecting both pollutant removal and energy consumption, which are directly related to the overall operating cost24 . Sodium chloride is commonly used to enhance the electrical conductivity of treated water and wastewater25, 26. However, the presence of sulfate and carbonate ions in the solution can lead to the precipitation of Ca and Mg ions, resulting in the formation of an insulating layer on the electrode surface. This layer increases the potential difference between the electrodes, thereby decreasing the system efficiency27 . Daneshvar et al. demonstrated that an increase in NaCl concentration enhances the efficiency of contaminant removal. In this study, a primary factor contributing to the improved efficiency at elevated salt concentrations was the increase in electrical conductivity, which facilitated a greater current flow through the circuit under a constant potential.28.
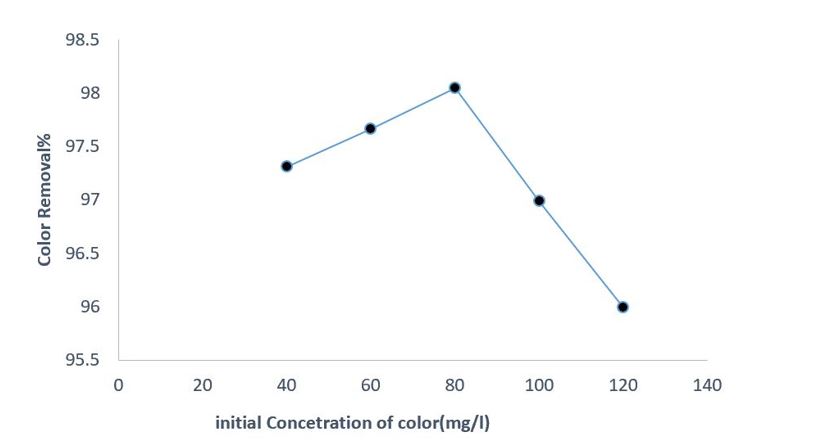
Effect of initial concentration of DR 89
At this stage, all operational conditions were adjusted to their optimum values, and the concentration of DR 89 was varied between 20 and 120 mg/L. As shown in Figure 6, the degradation efficiency of DR 89 using the EC/PS process increased from 97.30% to 98.04% as the dye concentration increased from 40 mg/L to 80 mg/L. However, increasing the concentration of the target contaminant above 80 mg/L decreased the removal efficiency.
Effect of initial concentration of DR 89
At this stage, all operational conditions were adjusted to their optimum values, and the concentration of DR 89 was varied between 20 and 120 mg/L. As shown in Figure 6, the degradation efficiency of DR 89 using the EC/PS process increased from 97.30% to 98.04% as the dye concentration increased from 40 mg/L to 80 mg/L. However, increasing the concentration of the target contaminant above 80 mg/L decreased the removal efficiency.

Figure 6: Effect of initial DR 89 concentration on removal efficiency by the EC-PS process (PH = 4, PS concentration = 25 mg/L, electrolyte concentration = 250 mg/L, current density = 1 mA/cm2, and reaction time = 25 min)
Discussion
The pH of an aqueous solution plays a crucial role in the decomposition of organic compounds in the environment. Based on these results, acidic conditions were more favorable for the removal of DR 89 than neutral and alkaline pH levels. This can be attributed to the enhanced production of sulfate radicals under acidic conditions, as shown in Equation (6-7).
H+ + S2O82- HS2O8- (6)
HS2O8- H+ + SO₄⁻•+SO42- (7)
The high concentration of protons in acidic media facilitates this process, thereby increasing the removal efficiency29. Moreover, the enhanced removal efficiency could be related to the increased generation of sulfate (SO₄⁻• −) and hydroxyl (•OH) radicals. It can be concluded that the sulfate and hydroxyl radicals produced have a higher oxidation potential under acidic conditions than under neutral and alkaline conditions 29.
As the pH increased, the removal efficiency gradually decreased, reaching 96.54% at pH 9.0. The decrease in removal efficiency at neutral and alkaline pH levels can be due to the precipitation of Fe3+ . When the pH exceeds 4.0, Fe3+ precipitation may occur30. In addition, at pH values > 4.0, the formation of iron complexes can hinder the reaction between iron and persulfate, thereby reducing the overall process efficiency. This mechanism is illustrated by the following reaction (Equation (8)):
Fe2+ + H2O FeOH+ + H+ (8)
Furthermore, the removal efficiency is further diminished due to the formation of iron oxyhydroxides, such as Fe(OH)24+ and Fe(OH)2+, which eventually transform into Fe(OH)3 at pH levels exceeding 4.0.31.
The reaction time directly influenced the number of ions generated by the iron electrodes. As the electrolysis time increased, the iron ion concentration also increased. This finding can be explained by Faraday's law, where the iron species as coagulants increase with time, which can initially lead to increased removal efficiency. However, prolonged electrolysis may lead to excessive accumulation of iron species, which can eventually hinder the process and reduce the efficiency32, 33. In a study conducted by Bazrafshan, utilizing iron electrodes in the electrocoagulation process, it was observed that the removal efficiency initially increased with prolonged reaction time.34. The application of an electric current by creating corrosion at the anode can lead to the continuous production of iron ions in the solution, which subsequently activates persulfate and generates sulfate radicals. Increasing the current density can result in faster and greater production of ferrous ions and promote the regeneration of ferric ions, which improves the degradation of persulfate and ultimately increases the removal of DR 8935.
However, further increasing the current density led to a decrease in the removal efficiency, which reached 95.25% at a current intensity of 2.37 mA/cm2. This reduction can be attributed to the excessive production of Fe2+ ions and their accumulation in the solution. Therefore, these ions may act as scavengers of SO₄•⁻ and ultimately reduce the removal efficiency, as shown in Equation (9) 36.
Fe2++SO₄•⁻ SO42- +Fe3+ (9)
A similar trend was reported by Akbari et al., who removed bisphenols using an electro-sulfate process. They observed that the removal efficiency initially increased with increasing current density and then decreased at higher current density37.
One of the key factors influencing free radical production for contaminant degradation is the oxidant concentration. As the oxidant concentration increased, the production of SO₄•⁻ accelerated, thereby enhancing the decomposition rate of DR 89.The study conducted by Zhang et al. utilizing the electro/Fe2+/peroxydisulfate process demonstrated an increasing trend in removal efficiency with elevated persulfate concentrations. 38. However, increasing the persulfate concentration beyond a certain amount leads to a side reaction between sulfate radicals and excess persulfate (Equation (10-11)). Finally, this process can lead to reduced removal efficiency38. A similar trend was reported by Zhang et al., who found that an overdose of persulfate during activation led to a decrease in the removal efficiency26.
SO₄•⁻ + SO₄•⁻ 2SO₄•⁻ (10)
S2O82- + SO₄•- S2O8•⁻ +SO42- (11)
Previous research has demonstrated that within the electrochemical process, the presence of a supporting electrolyte in the solution affects the current efficiency, cell voltage, and electrical energy consumption24, 39.
In addition, an increase in the organic matter concentration in the environment consumes more oxidants and prolongs the time required for complete degradation. Therefore, as the contaminant concentration increased, the process efficiency decreased slightly 40. Notably, intermediate compounds may form during oxidation at high concentrations. These compounds may act as scavengers of hydroxyl and sulfate radicals, thereby reducing the removal efficiency by creating competition between the contaminant molecules and intermediate compounds41, 42. In a similar study, Rahmani et al. reported that an increase in phenol concentration resulted in a reduction in the removal efficiency from 93.99 to 53 % for the EC/PS process. In this study, it was also stated that increasing the initial concentration of contaminants required more oxidation potential, and as a result, with a constant amount of oxidant, the process efficiency decreases43.
Conclusion
The EC/PS process is an AOPs capable of degrading environmentally persistent contaminants such as dyes. An investigation of the effective operational parameters, including persulfate concentration, current intensity, initial dye concentration, and initial pH, indicated that the highest removal efficiency (99.43%) was achieved under the following conditions: pH 4.0, reaction time 25 min, current density 1 mA/cm2, electrolyte concentration 250 mg/L, and persulfate concentration 30 mg/L for an aqueous solution containing 80 mg/L of DR89 dye. Owing to the very low biodegradation of azo dyes, the EC/PS process can be considered an effective process for the degradation of DR89 in a relatively short time. The approach used in this study is a simple and cost-effective method that provides rapid degradation of persistent organic compounds in an aqueous medium. Compared to other advanced oxidation processes, such as the use of nanoparticles, which may release emerging and sometimes toxic by-products into the environment, the introduced method is more environmentally friendly. Given its high efficiency and low environmental impact, the developed method can be extensively used for the treatment of industrial wastewater containing resistant organic pollutants.
Acknowledgements
The authors gratefully thank the deputy of Shahid Sadoughi University of Medical Sciences, for their financial support. The researchers did not receive any specific grants from funding agencies in the commercial or non-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research was funded by the Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number 20095).
Ethical Considerations
The authors affirm adherence to ethical standards in conducting this article, including compliance with publication ethics, avoidance of plagiarism, and accurate citation of original works. The study did not involve human or animal subjects directly.
Authors' Contributions
Arezo Rezaie and Behnam Hatami designed and edited the study; Arezo Rezaie conducted the experimental work; Maryam Malekbala analyzed the data; all authors discussed the results and contributed to the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Benkhaya S, M'rabet S, El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6(1):e03271.
2. Silveira JE, Garcia-Costa AL, Cardoso TO, et al. Indirect decolorization of azo dye Disperse Blue 3 by electro-activated persulfate. Electrochim Acta. 2017;258:927-32.
3. Martínez-Huitle CA, Brillas E. Decontamination of wasterwaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B. 2009;87:105-45.
4. Amani-Ghadim A, Aber S, Olad A, et al. Optimization of electrocoagulation process for removal of an azo dye using response surface methodology and investigation on the occurrence of destructive side reactions. Chemical Engineering and Processing: Process Intensification. 2013;64:68-78.
5. Daneshvar N, Salari D, Khataee A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A Chem. 2004;162(2-3):317-22.
6. Fallahzadeh RA, Mahvi AH, Meybodi MN, et al. Application of photo-electro oxidation process for amoxicillin removal from aqueous solution: modeling and toxicity evaluation. Korean J Chem Eng. 2019;36:713-21.
7. Samarghandi M, Shabanloo A, Shamsi K, et al. Performance of electrofenton process to remove cyanide from aquatic environments in presence of interfering humic acids. Journal of Health and Hygiene. 2014;4(4):293-303.
8. Samarghandi MR, Poureshgh Y, Vanaei Tabar M, et al. Investigating the Removal ethidium bromide from aqueous solutions using activated persulfate in electrochemical process. Pajouhan Scientific Journal. 2018;16(2):1-10.
9. Lin H, Zhang H, Hou L. Degradation of CI Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process. J Hazard Mater. 2014;276:182-91.
10. Lee J, Von Gunten U, Kim J-H. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ Sci Technol. 2020;54(6):3064-81.
11. Yang S, Wang P, Yang X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard Mater. 2010;179(1-3):552-8.
12. El-Dein AM, Libra J, Wiesmann U. Mechanism and kinetic model for the decolorization of the azo dye Reactive Black 5 by hydrogen peroxide and UV radiation. Chemosphere. 2003; 52(6):1069-77.
13. Gao J, Yang S, Li N, et al. Rapid degradation of azo dye Direct Black BN by magnetic MgFe2O4-SiC under microwave radiation. Appl Surf Sci. 2016;379:140-9.
14. Tauber MM, Guebitz GM, Rehorek A. Degradation of azo dyes by laccase and ultrasound treatment. Appl Environ Microbiol. 2005;71(5):2600-7.
15. Nematollahi D, Zohdijamil Z, Salehzadeh H. An efficient electrochemical method for the synthesis of N, N, N′, N′-tetraalkyl-4, 4′-azodianiline. Journal of Electroanalytical Chemistry. 2014;720:156-61.
16. Chen W-S, Huang C-P. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate. Chemosphere. 2015;125:175-81.
17. Liu J, Zhong S, Song Y, et al. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. Journal of Electroanalytical Chemistry. 2018;809:74-9.
18. Xu X-R, Li X-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep Purif Technol. 2010;72(1):105-11.
19. Long A, Zhang H. Selective oxidative degradation of toluene for the recovery of surfactant by an electro/Fe 2+/persulfate process. Environmental Science and Pollution Research. 2015; 22(15) : 11606-16.
20. Aseman-Bashiz E, Sayyaf H. Metformin degradation in aqueous solutions by electro-activation of persulfate and hydrogen peroxide using natural and synthetic ferrous ion sources. J Mol Liq. 2020;300:112285.
21. Epold I, Trapido M, Dulova N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chemical Engineering Journal. 2015;279:452-62.
22. Rezaie A, Ghaneian MT, Fatehizadeh A, et al. Synergistic degradation of triclosan from aqueous solution by combination of sulfate radical and electrocoagulation process. Desalination Water Treat. 2021;221:291-302.
23. Benabbas K, Hocini I, Khellaf N, editors. Biosorption of the anionic dye Direct Red 89 by the aquatic plant Callitriche obtusangula. InProceedings of the Third International Symposium on Materials and Sustainable Development 3; 2018: Springer; 2018.p.540-8.
24. Drouiche N, Aoudj S, Hecini M, et al. Study on the treatment of photovoltaic wastewater using electrocoagulation: fluoride removal with aluminium electrodes—characteristics of products. J Hazard Mater. 2009;169(1-3):65-9.
25. Liang ChenJu LC, Wang ZihSin WZ, Bruell C. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007; 66(1):106-13.
26. Yan S, Zhang X, Zhang H. Persulfate activation by Fe (III) with bioelectricity at acidic and near-neutral pH regimes: Homogeneous versus heterogeneous mechanism. J Hazard Mater. 2019; 374:92-100.
27. Fallahzadeh RA, Ehrampoush MH, Meybodi MN, et al. Investigating the effect of photo-electro oxidation process modified with activated carbon bed as a porous electrode on amoxicillin removal from aqueous solutions. Desalination Water Treat. 2020;185:185-95.
28. Daneshvar N, Ashassi-Sorkhabi H, Tizpar A. Decolorization of orange II by electrocoagulation method. Sep Purif Technol. 2003;31(2):153-62.
29. Jaafarzadeh N, Omidinasab M, Ghanbari F. Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Safety and Environmental Protection. 2016;102:462-72.
30. Xu X-R, Zhao Z-Y, Li X-Y, et al. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere. 2004;55(1):73-9.
31. Lin H, Wu J, Zhang H. Degradation of bisphenol A in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep Purif Technol. 2013;117:18-23.
32. Ahmad MK, Mohammed MA, Barbooti MM. Electrocoagulation for the removal of copper and zinc ions from water using iron electrodes. Open Chemistry Journal. 2020;7:37-43.
33. Bazrafshan E, Mahvi A, Nasseri S, et al. Removal of cadmium from industrial effluents by electrocoagulation process using iron electrodes. Iranian Journal of Environmental Health, Science & Engineering .2007; 3(4):261-6.
34. Bazrafshan E, Biglari H, Mahvi AH. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. J Chem. 2012;9 (4):2453-61.
35. Sepyani F, Soltani RDC, Jorfi S, et al. Implementation of continuously electro-generated Fe3O4 nanoparticles for activation of persulfate to decompose amoxicillin antibiotic in aquatic media: UV254 and ultrasound intensification. J Environ Manage. 2018;224:315-26.
36. Li J, Ren Y, Lai L, et al. Electrolysis assisted persulfate with annular iron sheet as anode for the enhanced degradation of 2, 4-dinitrophenol in aqueous solution. J Hazard Mater. 2018;344:778-87.
37. Akbari S, Ghanbari F, Moradi M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: applying low current density for oxidation mechanism. Chemical Engineering Journal. 2016;294:298-307.
38. Liang C, Wang Z-S, Bruell CJ. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007;66(1):106-13.
39. Fallahzadeha RA, Ehrampoushb MH, Meybodic MN, et al. Investigating the effect of photo-electro oxidation process modified with activated carbon bed as a porous electrode on amoxicillin removal from aqueous solutions. Desalination Water Treat. 2020;185:185-95.
40. Malakootian M, Asadi M. Efficiency of fenton oxidation process in removal of phenol in aqueous solutions. Journal of Water and Wastewater; Ab va Fazilab (in persian). 2011; 22(3):46-52.
41. Rahmani AR, Rezaeivahidian H, Almasi M, et al. A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Research on Chemical Intermediates. 2016;42(2):1441-50.
42. Peller JR, Cooper WJ, Ishida KP, et al. Evaluation of parameters influencing removal efficiencies for organic contaminant degradation in advanced oxidation processes. Journal of Water Supply: Research and Technology—AQUA. 2011;60(2):69-78.
43. Rahmani AR, Rezaeivahidian H, Almasi M, et al. A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Research on Chemical Intermediates. 2016;42:1441-50.
The pH of an aqueous solution plays a crucial role in the decomposition of organic compounds in the environment. Based on these results, acidic conditions were more favorable for the removal of DR 89 than neutral and alkaline pH levels. This can be attributed to the enhanced production of sulfate radicals under acidic conditions, as shown in Equation (6-7).
The high concentration of protons in acidic media facilitates this process, thereby increasing the removal efficiency29. Moreover, the enhanced removal efficiency could be related to the increased generation of sulfate (SO₄⁻• −) and hydroxyl (•OH) radicals. It can be concluded that the sulfate and hydroxyl radicals produced have a higher oxidation potential under acidic conditions than under neutral and alkaline conditions 29.
As the pH increased, the removal efficiency gradually decreased, reaching 96.54% at pH 9.0. The decrease in removal efficiency at neutral and alkaline pH levels can be due to the precipitation of Fe3+ . When the pH exceeds 4.0, Fe3+ precipitation may occur30. In addition, at pH values > 4.0, the formation of iron complexes can hinder the reaction between iron and persulfate, thereby reducing the overall process efficiency. This mechanism is illustrated by the following reaction (Equation (8)):
Furthermore, the removal efficiency is further diminished due to the formation of iron oxyhydroxides, such as Fe(OH)24+ and Fe(OH)2+, which eventually transform into Fe(OH)3 at pH levels exceeding 4.0.31.
The reaction time directly influenced the number of ions generated by the iron electrodes. As the electrolysis time increased, the iron ion concentration also increased. This finding can be explained by Faraday's law, where the iron species as coagulants increase with time, which can initially lead to increased removal efficiency. However, prolonged electrolysis may lead to excessive accumulation of iron species, which can eventually hinder the process and reduce the efficiency32, 33. In a study conducted by Bazrafshan, utilizing iron electrodes in the electrocoagulation process, it was observed that the removal efficiency initially increased with prolonged reaction time.34. The application of an electric current by creating corrosion at the anode can lead to the continuous production of iron ions in the solution, which subsequently activates persulfate and generates sulfate radicals. Increasing the current density can result in faster and greater production of ferrous ions and promote the regeneration of ferric ions, which improves the degradation of persulfate and ultimately increases the removal of DR 8935.
However, further increasing the current density led to a decrease in the removal efficiency, which reached 95.25% at a current intensity of 2.37 mA/cm2. This reduction can be attributed to the excessive production of Fe2+ ions and their accumulation in the solution. Therefore, these ions may act as scavengers of SO₄•⁻ and ultimately reduce the removal efficiency, as shown in Equation (9) 36.
A similar trend was reported by Akbari et al., who removed bisphenols using an electro-sulfate process. They observed that the removal efficiency initially increased with increasing current density and then decreased at higher current density37.
One of the key factors influencing free radical production for contaminant degradation is the oxidant concentration. As the oxidant concentration increased, the production of SO₄•⁻ accelerated, thereby enhancing the decomposition rate of DR 89.The study conducted by Zhang et al. utilizing the electro/Fe2+/peroxydisulfate process demonstrated an increasing trend in removal efficiency with elevated persulfate concentrations. 38. However, increasing the persulfate concentration beyond a certain amount leads to a side reaction between sulfate radicals and excess persulfate (Equation (10-11)). Finally, this process can lead to reduced removal efficiency38. A similar trend was reported by Zhang et al., who found that an overdose of persulfate during activation led to a decrease in the removal efficiency26.
Previous research has demonstrated that within the electrochemical process, the presence of a supporting electrolyte in the solution affects the current efficiency, cell voltage, and electrical energy consumption24, 39.
In addition, an increase in the organic matter concentration in the environment consumes more oxidants and prolongs the time required for complete degradation. Therefore, as the contaminant concentration increased, the process efficiency decreased slightly 40. Notably, intermediate compounds may form during oxidation at high concentrations. These compounds may act as scavengers of hydroxyl and sulfate radicals, thereby reducing the removal efficiency by creating competition between the contaminant molecules and intermediate compounds41, 42. In a similar study, Rahmani et al. reported that an increase in phenol concentration resulted in a reduction in the removal efficiency from 93.99 to 53 % for the EC/PS process. In this study, it was also stated that increasing the initial concentration of contaminants required more oxidation potential, and as a result, with a constant amount of oxidant, the process efficiency decreases43.
Conclusion
The EC/PS process is an AOPs capable of degrading environmentally persistent contaminants such as dyes. An investigation of the effective operational parameters, including persulfate concentration, current intensity, initial dye concentration, and initial pH, indicated that the highest removal efficiency (99.43%) was achieved under the following conditions: pH 4.0, reaction time 25 min, current density 1 mA/cm2, electrolyte concentration 250 mg/L, and persulfate concentration 30 mg/L for an aqueous solution containing 80 mg/L of DR89 dye. Owing to the very low biodegradation of azo dyes, the EC/PS process can be considered an effective process for the degradation of DR89 in a relatively short time. The approach used in this study is a simple and cost-effective method that provides rapid degradation of persistent organic compounds in an aqueous medium. Compared to other advanced oxidation processes, such as the use of nanoparticles, which may release emerging and sometimes toxic by-products into the environment, the introduced method is more environmentally friendly. Given its high efficiency and low environmental impact, the developed method can be extensively used for the treatment of industrial wastewater containing resistant organic pollutants.
Acknowledgements
The authors gratefully thank the deputy of Shahid Sadoughi University of Medical Sciences, for their financial support. The researchers did not receive any specific grants from funding agencies in the commercial or non-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research was funded by the Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number 20095).
Ethical Considerations
The authors affirm adherence to ethical standards in conducting this article, including compliance with publication ethics, avoidance of plagiarism, and accurate citation of original works. The study did not involve human or animal subjects directly.
Authors' Contributions
Arezo Rezaie and Behnam Hatami designed and edited the study; Arezo Rezaie conducted the experimental work; Maryam Malekbala analyzed the data; all authors discussed the results and contributed to the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Benkhaya S, M'rabet S, El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6(1):e03271.
2. Silveira JE, Garcia-Costa AL, Cardoso TO, et al. Indirect decolorization of azo dye Disperse Blue 3 by electro-activated persulfate. Electrochim Acta. 2017;258:927-32.
3. Martínez-Huitle CA, Brillas E. Decontamination of wasterwaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B. 2009;87:105-45.
4. Amani-Ghadim A, Aber S, Olad A, et al. Optimization of electrocoagulation process for removal of an azo dye using response surface methodology and investigation on the occurrence of destructive side reactions. Chemical Engineering and Processing: Process Intensification. 2013;64:68-78.
5. Daneshvar N, Salari D, Khataee A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A Chem. 2004;162(2-3):317-22.
6. Fallahzadeh RA, Mahvi AH, Meybodi MN, et al. Application of photo-electro oxidation process for amoxicillin removal from aqueous solution: modeling and toxicity evaluation. Korean J Chem Eng. 2019;36:713-21.
7. Samarghandi M, Shabanloo A, Shamsi K, et al. Performance of electrofenton process to remove cyanide from aquatic environments in presence of interfering humic acids. Journal of Health and Hygiene. 2014;4(4):293-303.
8. Samarghandi MR, Poureshgh Y, Vanaei Tabar M, et al. Investigating the Removal ethidium bromide from aqueous solutions using activated persulfate in electrochemical process. Pajouhan Scientific Journal. 2018;16(2):1-10.
9. Lin H, Zhang H, Hou L. Degradation of CI Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process. J Hazard Mater. 2014;276:182-91.
10. Lee J, Von Gunten U, Kim J-H. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ Sci Technol. 2020;54(6):3064-81.
11. Yang S, Wang P, Yang X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard Mater. 2010;179(1-3):552-8.
12. El-Dein AM, Libra J, Wiesmann U. Mechanism and kinetic model for the decolorization of the azo dye Reactive Black 5 by hydrogen peroxide and UV radiation. Chemosphere. 2003; 52(6):1069-77.
13. Gao J, Yang S, Li N, et al. Rapid degradation of azo dye Direct Black BN by magnetic MgFe2O4-SiC under microwave radiation. Appl Surf Sci. 2016;379:140-9.
14. Tauber MM, Guebitz GM, Rehorek A. Degradation of azo dyes by laccase and ultrasound treatment. Appl Environ Microbiol. 2005;71(5):2600-7.
15. Nematollahi D, Zohdijamil Z, Salehzadeh H. An efficient electrochemical method for the synthesis of N, N, N′, N′-tetraalkyl-4, 4′-azodianiline. Journal of Electroanalytical Chemistry. 2014;720:156-61.
16. Chen W-S, Huang C-P. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate. Chemosphere. 2015;125:175-81.
17. Liu J, Zhong S, Song Y, et al. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. Journal of Electroanalytical Chemistry. 2018;809:74-9.
18. Xu X-R, Li X-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep Purif Technol. 2010;72(1):105-11.
19. Long A, Zhang H. Selective oxidative degradation of toluene for the recovery of surfactant by an electro/Fe 2+/persulfate process. Environmental Science and Pollution Research. 2015; 22(15) : 11606-16.
20. Aseman-Bashiz E, Sayyaf H. Metformin degradation in aqueous solutions by electro-activation of persulfate and hydrogen peroxide using natural and synthetic ferrous ion sources. J Mol Liq. 2020;300:112285.
21. Epold I, Trapido M, Dulova N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chemical Engineering Journal. 2015;279:452-62.
22. Rezaie A, Ghaneian MT, Fatehizadeh A, et al. Synergistic degradation of triclosan from aqueous solution by combination of sulfate radical and electrocoagulation process. Desalination Water Treat. 2021;221:291-302.
23. Benabbas K, Hocini I, Khellaf N, editors. Biosorption of the anionic dye Direct Red 89 by the aquatic plant Callitriche obtusangula. InProceedings of the Third International Symposium on Materials and Sustainable Development 3; 2018: Springer; 2018.p.540-8.
24. Drouiche N, Aoudj S, Hecini M, et al. Study on the treatment of photovoltaic wastewater using electrocoagulation: fluoride removal with aluminium electrodes—characteristics of products. J Hazard Mater. 2009;169(1-3):65-9.
25. Liang ChenJu LC, Wang ZihSin WZ, Bruell C. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007; 66(1):106-13.
26. Yan S, Zhang X, Zhang H. Persulfate activation by Fe (III) with bioelectricity at acidic and near-neutral pH regimes: Homogeneous versus heterogeneous mechanism. J Hazard Mater. 2019; 374:92-100.
27. Fallahzadeh RA, Ehrampoush MH, Meybodi MN, et al. Investigating the effect of photo-electro oxidation process modified with activated carbon bed as a porous electrode on amoxicillin removal from aqueous solutions. Desalination Water Treat. 2020;185:185-95.
28. Daneshvar N, Ashassi-Sorkhabi H, Tizpar A. Decolorization of orange II by electrocoagulation method. Sep Purif Technol. 2003;31(2):153-62.
29. Jaafarzadeh N, Omidinasab M, Ghanbari F. Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Safety and Environmental Protection. 2016;102:462-72.
30. Xu X-R, Zhao Z-Y, Li X-Y, et al. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere. 2004;55(1):73-9.
31. Lin H, Wu J, Zhang H. Degradation of bisphenol A in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep Purif Technol. 2013;117:18-23.
32. Ahmad MK, Mohammed MA, Barbooti MM. Electrocoagulation for the removal of copper and zinc ions from water using iron electrodes. Open Chemistry Journal. 2020;7:37-43.
33. Bazrafshan E, Mahvi A, Nasseri S, et al. Removal of cadmium from industrial effluents by electrocoagulation process using iron electrodes. Iranian Journal of Environmental Health, Science & Engineering .2007; 3(4):261-6.
34. Bazrafshan E, Biglari H, Mahvi AH. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. J Chem. 2012;9 (4):2453-61.
35. Sepyani F, Soltani RDC, Jorfi S, et al. Implementation of continuously electro-generated Fe3O4 nanoparticles for activation of persulfate to decompose amoxicillin antibiotic in aquatic media: UV254 and ultrasound intensification. J Environ Manage. 2018;224:315-26.
36. Li J, Ren Y, Lai L, et al. Electrolysis assisted persulfate with annular iron sheet as anode for the enhanced degradation of 2, 4-dinitrophenol in aqueous solution. J Hazard Mater. 2018;344:778-87.
37. Akbari S, Ghanbari F, Moradi M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: applying low current density for oxidation mechanism. Chemical Engineering Journal. 2016;294:298-307.
38. Liang C, Wang Z-S, Bruell CJ. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007;66(1):106-13.
39. Fallahzadeha RA, Ehrampoushb MH, Meybodic MN, et al. Investigating the effect of photo-electro oxidation process modified with activated carbon bed as a porous electrode on amoxicillin removal from aqueous solutions. Desalination Water Treat. 2020;185:185-95.
40. Malakootian M, Asadi M. Efficiency of fenton oxidation process in removal of phenol in aqueous solutions. Journal of Water and Wastewater; Ab va Fazilab (in persian). 2011; 22(3):46-52.
41. Rahmani AR, Rezaeivahidian H, Almasi M, et al. A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Research on Chemical Intermediates. 2016;42(2):1441-50.
42. Peller JR, Cooper WJ, Ishida KP, et al. Evaluation of parameters influencing removal efficiencies for organic contaminant degradation in advanced oxidation processes. Journal of Water Supply: Research and Technology—AQUA. 2011;60(2):69-78.
43. Rahmani AR, Rezaeivahidian H, Almasi M, et al. A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Research on Chemical Intermediates. 2016;42:1441-50.
Type of Study: Original articles |
Subject:
Environmental Health, Sciences, and Engineering
Received: 2025/02/23 | Accepted: 2025/05/20 | Published: 2025/06/29
Received: 2025/02/23 | Accepted: 2025/05/20 | Published: 2025/06/29
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







