Volume 10, Issue 3 (September 2025)
J Environ Health Sustain Dev 2025, 10(3): 2781-2791 |
Back to browse issues page
Ethics code: IR.KMU.REC.1399.258
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nasab H, Hashemi M, Mirzaee M, Rajabi S, Ebrahimpour K. Enhanced Sensitive Determination of Triclosan and Methyl Triclosan in Human Urine by Dispersive Liquid–Liquid Microextraction Coupled with GC–MS. J Environ Health Sustain Dev 2025; 10 (3) :2781-2791
URL: http://jehsd.ssu.ac.ir/article-1-946-en.html
URL: http://jehsd.ssu.ac.ir/article-1-946-en.html
Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran & Department of Environmental Health Engineering, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran
Full-Text [PDF 682 kb]
(318 Downloads)
| Abstract (HTML) (302 Views)
Full-Text: (152 Views)
Habibeh Nasab 1, Majid Hashemi 2,3*, Moghaddameh Mirzaee 4, Saeed Rajabi 2,3, Karim Ebrahimpour 5
1 Environmental Sciences and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3 Department of Environmental Health Engineering, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran.
4 Modeling in Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran.
5 Department of Environmental Health Engineering, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran.
2 Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3 Department of Environmental Health Engineering, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran.
4 Modeling in Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran.
5 Department of Environmental Health Engineering, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Triclosan is a lipophilic antimicrobial agent primarily found in personal care products, including shampoos, toothpastes, and cosmetics. Both compounds are largely excreted in urine, making it the most accessible and reliable biological matrix for human exposure monitoring. This study aims to evaluate a novel extraction procedure dependent on the Dispersive Liquid–Liquid Microextraction (DLLME) technique to detect and quantify triclosan and methyl triclosan in urine samples from children and adolescents in Kerman. Materials and Methods: Triclosan and methyl triclosan levels in urine samples from 79 children and adolescents (6 to 18 years) in Kerman, Iran were measured by DLLME using the chromatography-mass -mass spectrometer (GC-MS) device. Results: The method yielded relative standard deviations (RSDs) of 4.6% and 3.9% for triclosan and methyl triclosan, respectively. The limits of detection (LOD) were 1.9 µg/L for triclosan and 1.8 µg/L for methyl triclosan, while the limits of quantification (LOQ) were 6.3 µg/L and 6.0 µg/L, respectively. The average urinary concentrations were 4.62 ± 2.08 µg/L for triclosan and 1.91 ± 0.88 µg/L for methyl triclosan. Conclusion: Triclosan and methyl triclosan were detected in all samples studied, indicating that all subjects were exposed to these compounds. These findings underscore the urgent need to reduce exposure pathways through enhanced public awareness and stringent regulatory oversight. |
|
Article History: Received: 10 June 2025 Accepted: 20 August 2025 |
||
*Corresponding Author: Majid Hashemi Email: mhashemi120@gmail.com Tel: +98 3431325075 |
||
Keywords: Biological Monitoring; Triclosan; Methyl Triclosan; Liquid Phase Microextraction. |
Citation: Nasab H, Hashemi M, Mirzaee M, et al. Enhanced Sensitive Determination of Triclosan and Methyl Triclosan in Human Urine by Dispersive Liquid–Liquid Microextraction Coupled with GC–MS. J Environ Health Sustain Dev. 2025; 10(3): 2781-91.
Introduction
Triclosan (2,4,4'-trichloro-2-hydroxy-diphenyl ether) is an antimicrobial compound categorized as a halogenated aromatic hydrocarbon, featuring the structural elements of phenols, diphenyl ethers, and polychlorinated biphenyls. Its halogenated biphenyl ether structure confers chemical features comparable to those of several toxic chemicals, including polychlorinated biphenyls (PCBs), bisphenol A (BPA), polybrominated diphenyl ethers (PBDEs), and dioxins 1-4. Triclosan is a broad-spectrum antimicrobial agent effective at low concentrations against gram-positive and gram-negative bacteria, as well as a wide range of viruses and fungi. Triclosan is a non-polar molecule with low water solubility. In addition to being resistant to hydrolysis, triclosan is very stable in acids 5, 6. Methyl triclosan is the primary metabolite of triclosan, formed by substituting the hydroxyl group with a methoxy group. The substitution of the hydroxyl group with a methoxy group results in the loss of antibacterial activity in methyl triclosan, while enhancing its lipid solubility, bioaccumulation potential, and chemical stability compared to triclosan 7. The widespread application of triclosan as an antibacterial ingredient in numerous products has raised concerns regarding its potential to promote bacterial resistance, which could extend to other antimicrobial agents. The European :union:’s Scientific Committee on Consumer Products has indicated that triclosan concentrations exceeding three percent in consumer products may present safety risks 8-10.
The use of triclosan has surged significantly over the past few decades. This rise in popularity is partly due to its ability to readily combine with various substances and its relatively high boiling point (280–290°C) 8, 11. Triclosan is used as a disinfectant, antiseptic, and preservative in a wide range of medical and personal care products, such as hand soaps, shampoos, conditioners, detergents, depilatory creams, toothpastes, mouthwashes, deodorants, antiperspirants, cosmetics, laundry products, fabric softeners, and acne treatments 12, 13. Triclosan can enter the body via various pathways, including skin absorption, ingestion, and inhalation, with ingestion and skin contact being the primary routes of exposure 14.
The extensive use of triclosan has raised concerns regarding its impact on human health 15. As an ethylbiphenyl chlorinated compound, triclosan is structurally similar to other halogenated chemicals like polychlorinated biphenyls, per-and polyfluoroalkyl substances (PFAS), bisphenols, and dioxins. Its aromatic nature and high chlorine content make it persistent in the environment and difficult to degrade. In laboratory animal models, evidence suggests that triclosan has negative effects on endocrine function, thyroid hormone homeostasis, and antibiotic resistance 16, 17. It also reduces the levels of progesterone and testosterone 18, 19, and triclosan has been linked to liver tumors 20. Human exposure to triclosan has been linked to asthma in children, increased cancer risk, and obesity 18. This substance can also change the composition or function of intestinal microflora 19.
Triclosan can be identified in various matrices, including urine, blood, breast milk, hair, nails, and amniotic fluid 15, 21. Urine is a non-invasive biomonitoring tool for measuring and assessing exposure to triclosan. Notably, triclosan is mainly excreted in urine and has a mean half-life of 21 h 8. A pharmacokinetic study found that following oral intake of four milligrams of triclosan, the majority of participants excreted the compound in their urine within 24 h, with levels returning to baseline within eight days. These findings suggest that urinary triclosan concentration is a reliable biomarker of triclosan exposure 16.
Therefore, developing a straightforward, rapid, efficient, and sensitive method for detecting triclosan and methyl triclosan in urine is crucial. Accurately quantifying these analytes is challenging because they are present at low concentrations in human samples. Therefore, appropriate extraction and purification procedures must be performed before determining the tool. Several pretreatment methods have been developed for triclosan and methyl triclosan, including solid-phase microextraction (SPME), liquid-liquid extraction (LLE), solid-phase extraction (SPE), and dispersive liquid-liquid microextraction (DLLME). Apart from DLLME, the drawbacks of previously studied procedures are evident, such as being time-consuming, expensive, and requiring the use of environmentally unfriendly solvents. The prominent advantages of DLLME include the requirement of very small extraction volumes and dispersing solvents, as well as short extraction times. Consequently, ionic liquid-based microextraction techniques have gained popularity in recent years 22. Since its introduction in 2006, this method has been extensively employed to detect trace amounts of various compounds across a range of matrices, including water, vegetables, soil, red wine, honey, human blood, hair, and
urine 23-25.
Numerous studies have examined the detection of triclosan and methyl triclosan, including research by Chu et al., who measured triclosan and triclocarban concentrations in sludge and biosolids from a municipal wastewater treatment plant using LC/MS with electrospray ionization (ESI) 26. Gatido et al. conducted a study utilizing a silyl-derivative GC/MS technique to analyze triclosan and bisphenol A concentrations in wastewater and sewage sludge 27. Liu et al. conducted a study assessing the relationship between parabens, triclosan, and benzophenones in urine samples and blood pressure during pregnancy, employing the DLLME method 28. Moreover, Hashemi et al. used DLLME with GC-MS to examine the levels of phthalates in the urine samples of children and adolescents 29. However, few studies have investigated the levels of triclosan and methyl triclosan in urine using DLLME combined with GC-MS. Although numerous studies have established the presence of triclosan and its metabolites in environmental media and biological fluids using various analytical techniques, limited attention has been given to the concurrent determination of triclosan and methyl triclosan in human urine using DLLME combined with GC-MS. This gap is particularly relevant for children and adolescents, who may exhibit different exposure patterns and physiological susceptibilities. In addition, no published data are available for the Iranian population, whose environmental exposure profiles may differ from those in other regions. The novelty of the present study lies in the application of DLLME-GC/MS for the concurrent detection of triclosan and methyl triclosan in urine samples from children and adolescents, representing the first biomonitoring report of this type in Iran. Accordingly, this study aimed to identify and quantify triclosan and methyl triclosan in urine samples collected from children and adolescents in Kerman City using DLLME-GC/MS.
Materials and Methods
Study population
A total of 79 urine samples were randomly collected in 2019 from children and adolescents aged 6–18 years who visited hospital laboratories in Kerman. Before sample collection, the participants were fully informed about the study, and written informed consent was obtained from their parents or guardians. All urine samples were stored at –20 °C until analysis 30.
Chemicals
The chemicals used in this study included hydrochloric acid, methyl tert-butyl ether
(MTBE), hexane, and N-trimethylsilyl-N-methyl trifluoroacetamide (MSTFA). They were obtained from Merck (Germany) and Sigma-Aldrich.
Measurement of triclosan and methyl triclosan
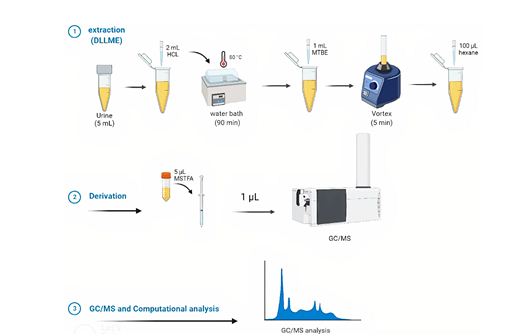
First, 2 ml of hydrochloric acid was added to 5 ml of urine in a Falcon tube, which was then heated in a steam bath at 80°C for 90 min. After the sample was cooled, 1 ml of MTBE solvent was added to it, then it was shaken for 5 min using a vortex shaker, and finally, 100 μl of hexane was added to it 31. The sample extracted from the previous step was transferred to a special vial for the chromatography-mass spectrometer (GC-MS) device, 5 µL of the MSTFA derivatizer was added to it, and then 1 µL of it was injected into the chromatography device . Gas chromatography–mass spectrometry (GC-MS, Agilent 7890 USA) coupled with a mass selective detector (Agilent 5975) featuring a split/splitless inlet and a quadrupole mass analyzer was utilized for analyzing samples. Separation was achieved on a silica-based capillary column (HP-5 MS; 5% phenyl–95% polydimethylsiloxane) 30 m long, with an interior diameter of 0.25 mm and a film thickness of 0.25 μm. A schematic overview of the analytical procedure is illustrated in Figure 1.
Triclosan (2,4,4'-trichloro-2-hydroxy-diphenyl ether) is an antimicrobial compound categorized as a halogenated aromatic hydrocarbon, featuring the structural elements of phenols, diphenyl ethers, and polychlorinated biphenyls. Its halogenated biphenyl ether structure confers chemical features comparable to those of several toxic chemicals, including polychlorinated biphenyls (PCBs), bisphenol A (BPA), polybrominated diphenyl ethers (PBDEs), and dioxins 1-4. Triclosan is a broad-spectrum antimicrobial agent effective at low concentrations against gram-positive and gram-negative bacteria, as well as a wide range of viruses and fungi. Triclosan is a non-polar molecule with low water solubility. In addition to being resistant to hydrolysis, triclosan is very stable in acids 5, 6. Methyl triclosan is the primary metabolite of triclosan, formed by substituting the hydroxyl group with a methoxy group. The substitution of the hydroxyl group with a methoxy group results in the loss of antibacterial activity in methyl triclosan, while enhancing its lipid solubility, bioaccumulation potential, and chemical stability compared to triclosan 7. The widespread application of triclosan as an antibacterial ingredient in numerous products has raised concerns regarding its potential to promote bacterial resistance, which could extend to other antimicrobial agents. The European :union:’s Scientific Committee on Consumer Products has indicated that triclosan concentrations exceeding three percent in consumer products may present safety risks 8-10.
The use of triclosan has surged significantly over the past few decades. This rise in popularity is partly due to its ability to readily combine with various substances and its relatively high boiling point (280–290°C) 8, 11. Triclosan is used as a disinfectant, antiseptic, and preservative in a wide range of medical and personal care products, such as hand soaps, shampoos, conditioners, detergents, depilatory creams, toothpastes, mouthwashes, deodorants, antiperspirants, cosmetics, laundry products, fabric softeners, and acne treatments 12, 13. Triclosan can enter the body via various pathways, including skin absorption, ingestion, and inhalation, with ingestion and skin contact being the primary routes of exposure 14.
The extensive use of triclosan has raised concerns regarding its impact on human health 15. As an ethylbiphenyl chlorinated compound, triclosan is structurally similar to other halogenated chemicals like polychlorinated biphenyls, per-and polyfluoroalkyl substances (PFAS), bisphenols, and dioxins. Its aromatic nature and high chlorine content make it persistent in the environment and difficult to degrade. In laboratory animal models, evidence suggests that triclosan has negative effects on endocrine function, thyroid hormone homeostasis, and antibiotic resistance 16, 17. It also reduces the levels of progesterone and testosterone 18, 19, and triclosan has been linked to liver tumors 20. Human exposure to triclosan has been linked to asthma in children, increased cancer risk, and obesity 18. This substance can also change the composition or function of intestinal microflora 19.
Triclosan can be identified in various matrices, including urine, blood, breast milk, hair, nails, and amniotic fluid 15, 21. Urine is a non-invasive biomonitoring tool for measuring and assessing exposure to triclosan. Notably, triclosan is mainly excreted in urine and has a mean half-life of 21 h 8. A pharmacokinetic study found that following oral intake of four milligrams of triclosan, the majority of participants excreted the compound in their urine within 24 h, with levels returning to baseline within eight days. These findings suggest that urinary triclosan concentration is a reliable biomarker of triclosan exposure 16.
Therefore, developing a straightforward, rapid, efficient, and sensitive method for detecting triclosan and methyl triclosan in urine is crucial. Accurately quantifying these analytes is challenging because they are present at low concentrations in human samples. Therefore, appropriate extraction and purification procedures must be performed before determining the tool. Several pretreatment methods have been developed for triclosan and methyl triclosan, including solid-phase microextraction (SPME), liquid-liquid extraction (LLE), solid-phase extraction (SPE), and dispersive liquid-liquid microextraction (DLLME). Apart from DLLME, the drawbacks of previously studied procedures are evident, such as being time-consuming, expensive, and requiring the use of environmentally unfriendly solvents. The prominent advantages of DLLME include the requirement of very small extraction volumes and dispersing solvents, as well as short extraction times. Consequently, ionic liquid-based microextraction techniques have gained popularity in recent years 22. Since its introduction in 2006, this method has been extensively employed to detect trace amounts of various compounds across a range of matrices, including water, vegetables, soil, red wine, honey, human blood, hair, and
urine 23-25.
Numerous studies have examined the detection of triclosan and methyl triclosan, including research by Chu et al., who measured triclosan and triclocarban concentrations in sludge and biosolids from a municipal wastewater treatment plant using LC/MS with electrospray ionization (ESI) 26. Gatido et al. conducted a study utilizing a silyl-derivative GC/MS technique to analyze triclosan and bisphenol A concentrations in wastewater and sewage sludge 27. Liu et al. conducted a study assessing the relationship between parabens, triclosan, and benzophenones in urine samples and blood pressure during pregnancy, employing the DLLME method 28. Moreover, Hashemi et al. used DLLME with GC-MS to examine the levels of phthalates in the urine samples of children and adolescents 29. However, few studies have investigated the levels of triclosan and methyl triclosan in urine using DLLME combined with GC-MS. Although numerous studies have established the presence of triclosan and its metabolites in environmental media and biological fluids using various analytical techniques, limited attention has been given to the concurrent determination of triclosan and methyl triclosan in human urine using DLLME combined with GC-MS. This gap is particularly relevant for children and adolescents, who may exhibit different exposure patterns and physiological susceptibilities. In addition, no published data are available for the Iranian population, whose environmental exposure profiles may differ from those in other regions. The novelty of the present study lies in the application of DLLME-GC/MS for the concurrent detection of triclosan and methyl triclosan in urine samples from children and adolescents, representing the first biomonitoring report of this type in Iran. Accordingly, this study aimed to identify and quantify triclosan and methyl triclosan in urine samples collected from children and adolescents in Kerman City using DLLME-GC/MS.
Materials and Methods
Study population
A total of 79 urine samples were randomly collected in 2019 from children and adolescents aged 6–18 years who visited hospital laboratories in Kerman. Before sample collection, the participants were fully informed about the study, and written informed consent was obtained from their parents or guardians. All urine samples were stored at –20 °C until analysis 30.
Chemicals
The chemicals used in this study included hydrochloric acid, methyl tert-butyl ether
(MTBE), hexane, and N-trimethylsilyl-N-methyl trifluoroacetamide (MSTFA). They were obtained from Merck (Germany) and Sigma-Aldrich.
Measurement of triclosan and methyl triclosan
First, 2 ml of hydrochloric acid was added to 5 ml of urine in a Falcon tube, which was then heated in a steam bath at 80°C for 90 min. After the sample was cooled, 1 ml of MTBE solvent was added to it, then it was shaken for 5 min using a vortex shaker, and finally, 100 μl of hexane was added to it 31. The sample extracted from the previous step was transferred to a special vial for the chromatography-mass spectrometer (GC-MS) device, 5 µL of the MSTFA derivatizer was added to it, and then 1 µL of it was injected into the chromatography device . Gas chromatography–mass spectrometry (GC-MS, Agilent 7890 USA) coupled with a mass selective detector (Agilent 5975) featuring a split/splitless inlet and a quadrupole mass analyzer was utilized for analyzing samples. Separation was achieved on a silica-based capillary column (HP-5 MS; 5% phenyl–95% polydimethylsiloxane) 30 m long, with an interior diameter of 0.25 mm and a film thickness of 0.25 μm. A schematic overview of the analytical procedure is illustrated in Figure 1.

Figure 1: Graphical summary of triclosan and methyl triclosan measurements in a urine sample
Perfluorotributylamine (PFTBA) was used to calibrate the mass spectrometer. Selected Ion Monitoring (SIM) analysis was conducted for each compound under study. Rather than scanning the entire range of mass-to-charge ratios (m/z), the instrument focuses only on specific m/z values with the highest intensity, as predetermined by the user. This targeted approach enhances sensitivity and is ideal for quantitative analyses. Consequently, to improve the detection sensitivity and precisely analyze the triclosan metabolites, the most abundant ions identified by the SIM program were selected, as detailed in Table 1.
Table 1: Selected key ions used for the quantitative analysis of triclosan and methyl triclosan
| Ion quantification (m/z) | Analytes |
| 161, 174, 198, 219, 253, and 302 | Triclosan |
| Methyl triclosan |
High-purity helium gas (99.99%) was employed as the mobile phase gas at a flow rate of 11 mL/min. To achieve optimal separation and resolution of the chromatographic peaks, various column temperature programs and carrier gas inlet flow rates were tested, as detailed in Table 2.
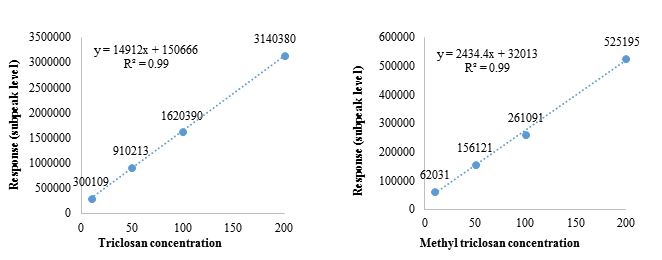
The input mode was splitless. MSD ChemStation software (ver. E.02.01.1177) 29, 32, 33 was used. Figure 2 shows the calibration curves of triclosan and methyl triclosan, and the coefficient of determination (R2) of the analytes was 0.99, which is close to 1.
The input mode was splitless. MSD ChemStation software (ver. E.02.01.1177) 29, 32, 33 was used. Figure 2 shows the calibration curves of triclosan and methyl triclosan, and the coefficient of determination (R2) of the analytes was 0.99, which is close to 1.
Table 2: Temperature schedule for triclosan and methyl triclosan analytes
| Injection amount | 1µl |
| Injection type | Splitless |
| Injection temperature | 290 °C |
| Column temperature program | At 60 °C for 2 minutes with a temperature gradient of 6 °C/minute to 280°C and holding at this temperature for 2 minutes |
| Helium gas flow | 1 mL/minute |
| Transmission line temperature | 290 °C |
| The source and quadrupole temperatures were considered at 230°C and 150°C, respectively. | |

Figure 2: Calibration curve of triclosan and methyl triclosan
Data Analysis
SPSS software was utilized for data analysis to assess the analytical performance of the proposed procedure. Precision was evaluated by computing the relative standard deviation (RSD), expressed as the percentage ratio of the standard deviation to the average of the repeated measurements. Furthermore, both intra-day and inter-day precision were determined by analyzing replicate spiked samples at various concentration levels. The standard deviation method based on S03 and S010 was used to compute the limit of detection (LOD) and quantification (LOQ), according to current analytical guidelines. The lowest concentration was defined by the LOD, yielding a signal-to-noise ratio of 3, whereas the LOQ corresponded to a signal-to-noise ratio of 10. Method linearity was assessed by constructing calibration curves and calculating R2.
Results
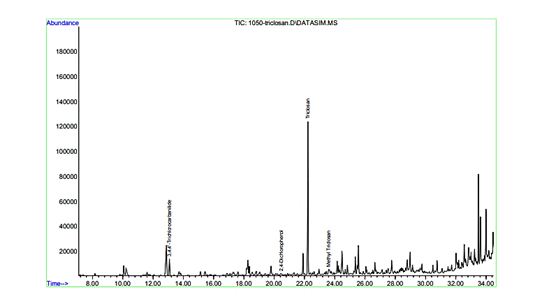
DLLME was used to extract triclosan and methyl triclosan analytes from biological samples (urine matrix). The factors affecting the dispersive liquid-liquid microextraction method include extraction time and solvent volume. Studies were conducted to select the optimal extraction conditions and appropriate solvent (MTBE), extractant, and dispersant (chlorobenzene)29. Typical chromatograms output from the GC/MS device for the analytes under investigation are shown in Figure 3. The chromatogram exhibits well-resolved and identifiable peaks for triclosan and methyl triclosan, confirming the method's selectivity, efficiency, and reliability for the concurrent determination of the target analytes.
Validation
The validation results are presented in Table 3. Following the establishment of optimal conditions, the detection limit and R2 were measured to evaluate the performance of the dispersive liquid-liquid microextraction procedure. As mentioned in Table 3, the R2 values ranged from 0.997 to 0.999, indicating a strong correlation. The LOD and LOQ were calculated using S03 and S010 29. The detection and measurement limits are presented in Table 3. Accordingly, the detection limit and quantification limit were 6.0-1.8 and 6.3-1.9 micrograms/liter for triclosan and methyl triclosan, respectively. The results show that the RSD is between 3.9 and 6.4%, which indicates the high accuracy of the experiments. The reproducibility of the proposed method meets the precise requirements of practical diagnosis.
SPSS software was utilized for data analysis to assess the analytical performance of the proposed procedure. Precision was evaluated by computing the relative standard deviation (RSD), expressed as the percentage ratio of the standard deviation to the average of the repeated measurements. Furthermore, both intra-day and inter-day precision were determined by analyzing replicate spiked samples at various concentration levels. The standard deviation method based on S03 and S010 was used to compute the limit of detection (LOD) and quantification (LOQ), according to current analytical guidelines. The lowest concentration was defined by the LOD, yielding a signal-to-noise ratio of 3, whereas the LOQ corresponded to a signal-to-noise ratio of 10. Method linearity was assessed by constructing calibration curves and calculating R2.
Results
DLLME was used to extract triclosan and methyl triclosan analytes from biological samples (urine matrix). The factors affecting the dispersive liquid-liquid microextraction method include extraction time and solvent volume. Studies were conducted to select the optimal extraction conditions and appropriate solvent (MTBE), extractant, and dispersant (chlorobenzene)29. Typical chromatograms output from the GC/MS device for the analytes under investigation are shown in Figure 3. The chromatogram exhibits well-resolved and identifiable peaks for triclosan and methyl triclosan, confirming the method's selectivity, efficiency, and reliability for the concurrent determination of the target analytes.
Validation
The validation results are presented in Table 3. Following the establishment of optimal conditions, the detection limit and R2 were measured to evaluate the performance of the dispersive liquid-liquid microextraction procedure. As mentioned in Table 3, the R2 values ranged from 0.997 to 0.999, indicating a strong correlation. The LOD and LOQ were calculated using S03 and S010 29. The detection and measurement limits are presented in Table 3. Accordingly, the detection limit and quantification limit were 6.0-1.8 and 6.3-1.9 micrograms/liter for triclosan and methyl triclosan, respectively. The results show that the RSD is between 3.9 and 6.4%, which indicates the high accuracy of the experiments. The reproducibility of the proposed method meets the precise requirements of practical diagnosis.

Figure 3: An example of a typical chromatogram for the identification of triclosan and methyl triclosan from the GC/MS device output
Table 3: Validation results of DLLME method.
| Analytes | LOD (μg/L) | LOQ (μg/L) | RSD (%) | R2 | Regression equation |
| Triclosan | 1.8 | 6.0 | 4.6 | 0.99 | Y=14912X + 150666 |
| Methyl triclosan | 1.9 | 6.3 | 3.9 | 0.99 | Y=2434.4X + 32013 |
| 1. Limit of detection (LOD) 2. Limits of quantification (LOQ) 3. Relative standard deviation (RSD) 4. Determination (R2) |
|||||
Table 4 shows the mean, minimum, and maximum concentrations of urinary triclosan metabolites in the study population (children and adolescents). This table shows the mean concentrations of metabolites in the three tertiles (data divided into three equal parts). The mean concentrations of triclosan and methyl triclosan were 4.62 ± 2.08 and 1.91 ± 0.88 (μg/L), respectively.
Table 4: Measured concentrations of triclosan metabolites
| Analytes | N | Mean ± SD | Minimum | Maximum |
| Triclosan (μg/L) | 79 | 4.62 ± 2.08 | 0.55 | 19.90 |
| Methyl triclosan (μg/L) | 79 | 1.91 ± 0.88 | 0.60 | 6.72 |
Discussion
The present study investigated a DLLME-based extraction procedure for the detection and quantification of triclosan and methyltriclosan in urine samples from children and adolescents and provided insights into the analytical performance of this technique and the exposure level of the target population. The findings of the present study demonstrated that the average concentrations of triclosan and methyl triclosan were 4.62 ± 2.08 and 1.91 ± 0.88 (μg/L), respectively. In 2017, Iyer et al. examined triclosan levels in a population of children and adolescents aged 10–19 years, and their results showed that the geometric mean of triclosan was 11.3 μg/L18. Rocha et al. (2018) examined triclosan levels in children and adolescents aged 6 to 14 and found that the geometric average triclosan level was 26.8 μg/L 23. This is almost double the level reported in this study. Spanier et al. (2014) examined the level of triclosan in a population of children aged 6 to 18 and found a geometric mean of 15.5 μg/L 39, which is approximately 3.5 times higher than the findings of the present study. The chronic reference dose (RfD) for triclosan is 0.03 mg/kg/day, according to the U.S. Environmental Protection Agency 40. Biomonitoring data from the National Health and Nutrition Examination Survey (NHANES) reported mean urinary triclosan concentrations of approximately 13 µg/L in the U.S. population 41. In comparison, the concentrations observed in this study were within or lower than this range, indicating a relatively low exposure in the study population. Nevertheless, the potential for endocrine disruption, bioaccumulation, and environmental toxicity has led regulatory agencies, such as the European Commission and Health Canada, to ban or limit triclosan in some applications 42, 43, which justifies the recommendation to limit human exposure, even at low detection levels.
To critically assess the performance of the developed procedure, a comparative analysis was conducted with several established analytical techniques, as summarized in Table 5. These include coupled solid-phase extraction and
ultra-performance liquid chromatography–mass spectrometry (SPE-UPLC/MS), liquid–solid extraction followed by gas chromatography–
mass spectrometry (LSE-GC/MS), solid-phase extraction incorporated with high-performance liquid chromatography–mass spectrometry (SPE-HPLC/MS), liquid–liquid extraction paired with high-performance liquid chromatography–tandem mass spectrometry (LLE-HPLC/MS/MS), air-assisted liquid–liquid microextraction combined with liquid chromatography–tandem mass spectrometry (AALLME-LC/MS/MS), ionic liquid-based solid–liquid microextraction coupled with high-performance liquid chromatography–ultraviolet detection (HPLC-UV), and dispersive liquid–liquid microextraction followed by capillary zone electrophoresis with ultraviolet detection (DLLME-CZE/UV)22.
The DLLME-GC/MS method employed in this study has several notable advantages over previously reported techniques. The total sample preparation time was significantly reduced, as no enzymatic hydrolysis or extended incubation was required, unlike methods such as SPE or LLE, which often involve pretreatment steps lasting
8–48 h. 34, 35. Enhanced repeatability and analytical precision were achieved, as reflected in the lower RSD values of 3.9–4.6%, compared to those reported for SPE (9%) and LSE (5.2–10.5%) 35, 36. In addition, the reduced consumption of organic solvents contributes to greater environmental sustainability and lower operational costs. Although the limit of detection (1.8–1.9 µg/L) was not the lowest among the evaluated techniques, it was deemed adequate for trace-level detection in biological and environmental matrices. The use of GC/MS enabled high sensitivity and selectivity for non-polar compounds such as triclosan and methyl triclosan. Overall, the DLLME-GC/MS method offers a practical balance of analytical performance, operational simplicity, and environmental friendliness, making it well-suited for biomonitoring applications, particularly in large-scale or resource-constrained settings.
The present study investigated a DLLME-based extraction procedure for the detection and quantification of triclosan and methyltriclosan in urine samples from children and adolescents and provided insights into the analytical performance of this technique and the exposure level of the target population. The findings of the present study demonstrated that the average concentrations of triclosan and methyl triclosan were 4.62 ± 2.08 and 1.91 ± 0.88 (μg/L), respectively. In 2017, Iyer et al. examined triclosan levels in a population of children and adolescents aged 10–19 years, and their results showed that the geometric mean of triclosan was 11.3 μg/L18. Rocha et al. (2018) examined triclosan levels in children and adolescents aged 6 to 14 and found that the geometric average triclosan level was 26.8 μg/L 23. This is almost double the level reported in this study. Spanier et al. (2014) examined the level of triclosan in a population of children aged 6 to 18 and found a geometric mean of 15.5 μg/L 39, which is approximately 3.5 times higher than the findings of the present study. The chronic reference dose (RfD) for triclosan is 0.03 mg/kg/day, according to the U.S. Environmental Protection Agency 40. Biomonitoring data from the National Health and Nutrition Examination Survey (NHANES) reported mean urinary triclosan concentrations of approximately 13 µg/L in the U.S. population 41. In comparison, the concentrations observed in this study were within or lower than this range, indicating a relatively low exposure in the study population. Nevertheless, the potential for endocrine disruption, bioaccumulation, and environmental toxicity has led regulatory agencies, such as the European Commission and Health Canada, to ban or limit triclosan in some applications 42, 43, which justifies the recommendation to limit human exposure, even at low detection levels.
To critically assess the performance of the developed procedure, a comparative analysis was conducted with several established analytical techniques, as summarized in Table 5. These include coupled solid-phase extraction and
ultra-performance liquid chromatography–mass spectrometry (SPE-UPLC/MS), liquid–solid extraction followed by gas chromatography–
mass spectrometry (LSE-GC/MS), solid-phase extraction incorporated with high-performance liquid chromatography–mass spectrometry (SPE-HPLC/MS), liquid–liquid extraction paired with high-performance liquid chromatography–tandem mass spectrometry (LLE-HPLC/MS/MS), air-assisted liquid–liquid microextraction combined with liquid chromatography–tandem mass spectrometry (AALLME-LC/MS/MS), ionic liquid-based solid–liquid microextraction coupled with high-performance liquid chromatography–ultraviolet detection (HPLC-UV), and dispersive liquid–liquid microextraction followed by capillary zone electrophoresis with ultraviolet detection (DLLME-CZE/UV)22.
The DLLME-GC/MS method employed in this study has several notable advantages over previously reported techniques. The total sample preparation time was significantly reduced, as no enzymatic hydrolysis or extended incubation was required, unlike methods such as SPE or LLE, which often involve pretreatment steps lasting
8–48 h. 34, 35. Enhanced repeatability and analytical precision were achieved, as reflected in the lower RSD values of 3.9–4.6%, compared to those reported for SPE (9%) and LSE (5.2–10.5%) 35, 36. In addition, the reduced consumption of organic solvents contributes to greater environmental sustainability and lower operational costs. Although the limit of detection (1.8–1.9 µg/L) was not the lowest among the evaluated techniques, it was deemed adequate for trace-level detection in biological and environmental matrices. The use of GC/MS enabled high sensitivity and selectivity for non-polar compounds such as triclosan and methyl triclosan. Overall, the DLLME-GC/MS method offers a practical balance of analytical performance, operational simplicity, and environmental friendliness, making it well-suited for biomonitoring applications, particularly in large-scale or resource-constrained settings.
Table 5: Comparison of the DLLME method with GC.MS device with other procedures
Conclusion
In this study, DLLME coupled with GC-MS was successfully applied to analyze triclosan and methyl triclosan in urine samples. This approach demonstrated good reproducibility and recovery within a short timeframe. Compared to other extraction techniques, such as SPE, LSE, and DLLME, the CZE-UV method exhibited a lower RSD, highlighting its superior accuracy. The average concentrations of triclosan and methyl triclosan in children and adolescents were 4.62 and 1.91 μg/L, respectively, demonstrating that children and adolescents are exposed to triclosan and that there is a need to limit the routes of exposure to triclosan.
Acknowledgments
This study was approved by the IR.KMU.REC.1399.258 ethical approval code and was conducted at the Environmental Health Engineering Research Center of Kerman University of Medical Sciences. The authors would like to thank the Vice-Chancellor for Research and Technology of the Kerman University of Medical Sciences.
Conflict of Interests
The authors declared no conflicts of interest.
Funding
This research was conducted in the Environmental Health Engineering Research Center of Kerman University of Medical Sciences. This research was supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences.
Ethical Considerations
The authors obtained permission from the Ethics Committee of the Kerman University of Medical Sciences.
Code of Ethics
The present study has the code of ethics IR.KMU.REC.1399.258 of Kerman University of Medical Sciences, Kerman, Iran.
Authors' Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by H.N, M.H, M.M, S.R, and K.E. The first draft of the manuscript was written by H.N, M.H and S.R, and all the authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization was performed by H.N; methodology by HN and K.E; formal analysis and investigation by M.M; Writing-original draft by H.N, and S.R; and writing-review and editing were done by M.H and K.E.
Ethical Issues
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Dhillon GS, Kaur S, Pulicharla R, et al. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015;12(5):5657-84.
2. Montaseri H, Forbes PB. A review of monitoring methods for triclosan and its occurrence in aquatic environments. Trends Analyt Chem. 2016;85:221-31.
3. Nasab H, Mirzaee M, Hashemi M, et al. Measurement of urinary Triclocarban and 2, 4-Dichlorophenol concentration and their relationship with obesity and predictors of cardiovascular diseases among children and adolescents in Kerman, Iran. J Environ Public Health. 2022;2022(1):2939022.
4. Rezaie A, Ghaneian MT, Fatehizadeh A, et al. Synergistic degradation of triclosan from aqueous solution by combination of sulfate radical and electrocoagulation process. Desalination Water Treat. 2021;221:291-302.
5. Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: a critical review of the literature. Crit Rev Toxicol. 2010;40(sup3):1-30.
6. Nguyen HT, Isobe T, Iwai-Shimada M, et al. Urinary concentrations and elimination half-lives of parabens, benzophenones, bisphenol and triclosan in Japanese young adults. Chemosphere. 2024;349:140920.
7. Li X, Shang Y, Yao W, et al. Comparison of transcriptomics changes induced by TCS and MTCS exposure in human hepatoma HepG2 cells. ACS Omega. 2020;5(19):10715-24.
8. Giuliano CA, Rybak MJ. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: a focused review. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015;35(3):328-36.
9. Dong M, Xu X, Huang Q, et al. Dose-dependent effects of triclocarban exposure on lipid homeostasis in rats. Chem Res Toxicol. 2019;32(11):2320-8.
10. Nasab H, Rajabi S, Mirzaee M, et al. Association of urinary triclosan, methyl triclosan, triclocarban, and 2, 4-dichlorophenol levels with anthropometric and demographic parameters in children and adolescents in 2020 (case study: Kerman, Iran). Environ Sci Pollut Res Int. 2022;29(20): 30754-63.
11. Pan Y, Wei X, Zhu Z, et al. Co-exposure of Parabens, Benzophenones, Triclosan, and Triclocarban in human urine from children and adults in South China. Chemosphere. 2024;363: 142936.
12. Goodman M, Naiman DQ, LaKind JS. Systematic review of the literature on triclosan and health outcomes in humans. Crit Rev Toxicol. 2018;48(1):1-51.
13. Levasseur JL, Hoffman K, Zhang S, et al. Monitoring human exposure to four parabens and triclosan: comparing silicone wristbands with spot urine samples as predictors of internal dose. J Expo Sci Environ Epidemiol. 2024;34(4):670-8.
14. Plumlee K. Clinical veterinary toxicology-E-Book: Elsevier Health Sciences; 2003.
15. Karzi V, Tzatzarakis MN, Vakonaki E, et al. Biomonitoring of Bisphenol A, Triclosan and Perfluorooctanoic Acid in hair samples of children and adults. J Appl Toxicol. 2018;38(8):1144-52.
16. Yueh M-F, Tukey RH. Triclosan: a widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol. 2016;56(1):251-72.
17. Pezeshki H, Rajabi S, Hashemi M, et al. Per-and poly-fluoroalkyl substances as forever chemicals in drinking water: unraveling the nexus with obesity and endocrine disruption–A mini review. Heliyon. 2025.
18. Iyer AP, Xue J, Honda M, et al. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: association with oxidative stress. Environmental Research. 2018;160:91-6.
19. Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. Journal of Toxicology and Environmental Health, Part B. 2017;20(8):447-69.
20. Park M, Kim S, Kim Y, et al. Relationship between personal care products usage and triclosan exposure: the second Korean National Environmental Health Survey (KoNEHS 2012–2014). Ann Occup Environ Med. 2019;31:1-8.
21. Li S, Zhao J, Wang G, et al. Urinary Triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: experience in NHANES 2003–2010. Int J Hyg Environ Health. 2015;218(4):401-6.
22. Wang X, Gao M, Gao J, et al. Extraction of Triclosan and Methyltriclosan in human fluids by in situ ionic liquid morphologic transformation. Journal of Chromatography B. 2018;1092:19-28.
23. Wang H, Zhang A, Wang W, et al. Separation and determination of triclosan and Bisphenol A in water, beverage, and urine samples by dispersive liquid–liquid microextraction combined with capillary zone electrophoresis–UV detection. J AOAC Int. 2013;96(2):459-65.
24. Hesaruiyeh FA, Rajabi S, Motamed-Jahromi M, et al. A pilot study on the association of Lead, 8-Hydroxyguanine, and Malondialdehyde levels in opium addicts’ blood serum with illicit drug use and non-addict persons. Int J Environ Res Public Health. 2022;19(15):9110.
25. Khademi N, Rajabi S, Fararouei M, et al. Environmental exposure to organophosphate pesticides and effects on cognitive functions in elementary school children in a Middle Eastern area. Environ Sci Pollut Res Int. 2023;30(51):111076-91.
26. Chu S, Metcalfe CD. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2007;1164(1-2):212-8.
27. Gatidou G, Thomaidis NS, Stasinakis AS, et al. Simultaneous determination of the endocrine disrupting compounds nonylphenol, nonylphenol ethoxylates, triclosan and bisphenol A in wastewater and sewage sludge by gas chromatography–mass spectrometry. J Chromatogr A. 2007;1138(1-2):32-41.
28. Liu H, Li J, Xia W, et al. Blood pressure changes during pregnancy in relation to urinary paraben, triclosan and benzophenone concentrations: a repeated measures study. Environ Int. 2019;122:185-92.
29. Amin MM, Ebrahimpour K, Parastar S, et al. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547-56.
30. Nasab H, Mirzaee M, Ebrahimpour K, et al. Association of urinary triclosan and methyl-triclosan levels with predictive indicators of cardiovascular disease and obesity in children and adolescents in 2020 (case study: Kerman, Iran). Environmental Health Engineering and Management Journal. 2021;8(3):187-95.
31. Rezaee M, Yamini Y, Faraji M. Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A. 2010;1217(16): 2342-57.
32. Cui Y, Shen N, Wang S, et al. Trace anti-inflammatory β-carboline alkaloid identified in Arenaria kansuensis by two-dimensional chromatography coupled with UniElut C18AEX based solid-phase extraction re-enrichment technology. Journal of Chromatography B. 2017;1068:282-8.
33. Hoogewerff JA, Reimann C, Ueckermann H, et al. Bioavailable 87Sr/86Sr in European soils: a baseline for provenancing studies. Sci Total Environ. 2019;672:1033-44.
34. Asimakopoulos AG, Wang L, Thomaidis NS, et al. A multi-class bioanalytical methodology for the determination of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters, benzophenone-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2014;1324:141-8.
35. Chen M, Zhu P, Xu B, et al. Determination of nine environmental phenols in urine by ultra-high-performance liquid chromatography–tandem mass spectrometry. J Anal Toxicol. 2012;36(9):608-15.
36. Pirard C, Sagot C, Deville M, et al. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ Int. 2012;48:78-83.
37. Gavin QW, Ramage RT, Waldman JM, et al. Development of HPLC-MS/MS method for the simultaneous determination of environmental phenols in human urine. Int J Environ Anal Chem. 2014; 94(2):168-82.
38. Rocha BA, de Oliveira ARM, Barbosa Jr F. A fast and simple air-assisted liquid-liquid microextraction procedure for the simultaneous determination of bisphenols, parabens, benzophenones, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. Talanta. 2018;183:94-101.
39. Spanier AJ, Fausnight T, Camacho TF, et al. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. InAllergy and asthma proceedings; 2014; 35(6):475. OceanSide Publications.
40. Morgan MK, Clifton MS. Exposure to triclosan and bisphenol analogues B, F, P, S and Z in repeated duplicate-diet solid food samples of adults. Toxics. 2021;9(3):47.
41. Calafat AM, Ye X, Wong L-Y, et al. Urinary concentrations of triclosan in the US population: 2003–2004. Environ Health Perspect. 2008;116(3):303-7.
42. Perez AL, Gauthier AM, Ferracini T, et al. The challenge of predicting problematic chemicals using a decision analysis tool: Triclosan as a case study. Integr Environ Assess Manag. 2016;13(1):198-207.
43. Cheng C, Zhou J, Liao J, et al. Investigation on the interactions of contaminant triclosan with human serum albumin: Spectroscopic and molecular docking studies. J Mol Struct. 2024;1295:136737.
In this study, DLLME coupled with GC-MS was successfully applied to analyze triclosan and methyl triclosan in urine samples. This approach demonstrated good reproducibility and recovery within a short timeframe. Compared to other extraction techniques, such as SPE, LSE, and DLLME, the CZE-UV method exhibited a lower RSD, highlighting its superior accuracy. The average concentrations of triclosan and methyl triclosan in children and adolescents were 4.62 and 1.91 μg/L, respectively, demonstrating that children and adolescents are exposed to triclosan and that there is a need to limit the routes of exposure to triclosan.
Acknowledgments
This study was approved by the IR.KMU.REC.1399.258 ethical approval code and was conducted at the Environmental Health Engineering Research Center of Kerman University of Medical Sciences. The authors would like to thank the Vice-Chancellor for Research and Technology of the Kerman University of Medical Sciences.
Conflict of Interests
The authors declared no conflicts of interest.
Funding
This research was conducted in the Environmental Health Engineering Research Center of Kerman University of Medical Sciences. This research was supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences.
Ethical Considerations
The authors obtained permission from the Ethics Committee of the Kerman University of Medical Sciences.
Code of Ethics
The present study has the code of ethics IR.KMU.REC.1399.258 of Kerman University of Medical Sciences, Kerman, Iran.
Authors' Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by H.N, M.H, M.M, S.R, and K.E. The first draft of the manuscript was written by H.N, M.H and S.R, and all the authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization was performed by H.N; methodology by HN and K.E; formal analysis and investigation by M.M; Writing-original draft by H.N, and S.R; and writing-review and editing were done by M.H and K.E.
Ethical Issues
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Dhillon GS, Kaur S, Pulicharla R, et al. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015;12(5):5657-84.
2. Montaseri H, Forbes PB. A review of monitoring methods for triclosan and its occurrence in aquatic environments. Trends Analyt Chem. 2016;85:221-31.
3. Nasab H, Mirzaee M, Hashemi M, et al. Measurement of urinary Triclocarban and 2, 4-Dichlorophenol concentration and their relationship with obesity and predictors of cardiovascular diseases among children and adolescents in Kerman, Iran. J Environ Public Health. 2022;2022(1):2939022.
4. Rezaie A, Ghaneian MT, Fatehizadeh A, et al. Synergistic degradation of triclosan from aqueous solution by combination of sulfate radical and electrocoagulation process. Desalination Water Treat. 2021;221:291-302.
5. Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: a critical review of the literature. Crit Rev Toxicol. 2010;40(sup3):1-30.
6. Nguyen HT, Isobe T, Iwai-Shimada M, et al. Urinary concentrations and elimination half-lives of parabens, benzophenones, bisphenol and triclosan in Japanese young adults. Chemosphere. 2024;349:140920.
7. Li X, Shang Y, Yao W, et al. Comparison of transcriptomics changes induced by TCS and MTCS exposure in human hepatoma HepG2 cells. ACS Omega. 2020;5(19):10715-24.
8. Giuliano CA, Rybak MJ. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: a focused review. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015;35(3):328-36.
9. Dong M, Xu X, Huang Q, et al. Dose-dependent effects of triclocarban exposure on lipid homeostasis in rats. Chem Res Toxicol. 2019;32(11):2320-8.
10. Nasab H, Rajabi S, Mirzaee M, et al. Association of urinary triclosan, methyl triclosan, triclocarban, and 2, 4-dichlorophenol levels with anthropometric and demographic parameters in children and adolescents in 2020 (case study: Kerman, Iran). Environ Sci Pollut Res Int. 2022;29(20): 30754-63.
11. Pan Y, Wei X, Zhu Z, et al. Co-exposure of Parabens, Benzophenones, Triclosan, and Triclocarban in human urine from children and adults in South China. Chemosphere. 2024;363: 142936.
12. Goodman M, Naiman DQ, LaKind JS. Systematic review of the literature on triclosan and health outcomes in humans. Crit Rev Toxicol. 2018;48(1):1-51.
13. Levasseur JL, Hoffman K, Zhang S, et al. Monitoring human exposure to four parabens and triclosan: comparing silicone wristbands with spot urine samples as predictors of internal dose. J Expo Sci Environ Epidemiol. 2024;34(4):670-8.
14. Plumlee K. Clinical veterinary toxicology-E-Book: Elsevier Health Sciences; 2003.
15. Karzi V, Tzatzarakis MN, Vakonaki E, et al. Biomonitoring of Bisphenol A, Triclosan and Perfluorooctanoic Acid in hair samples of children and adults. J Appl Toxicol. 2018;38(8):1144-52.
16. Yueh M-F, Tukey RH. Triclosan: a widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol. 2016;56(1):251-72.
17. Pezeshki H, Rajabi S, Hashemi M, et al. Per-and poly-fluoroalkyl substances as forever chemicals in drinking water: unraveling the nexus with obesity and endocrine disruption–A mini review. Heliyon. 2025.
18. Iyer AP, Xue J, Honda M, et al. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: association with oxidative stress. Environmental Research. 2018;160:91-6.
19. Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. Journal of Toxicology and Environmental Health, Part B. 2017;20(8):447-69.
20. Park M, Kim S, Kim Y, et al. Relationship between personal care products usage and triclosan exposure: the second Korean National Environmental Health Survey (KoNEHS 2012–2014). Ann Occup Environ Med. 2019;31:1-8.
21. Li S, Zhao J, Wang G, et al. Urinary Triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: experience in NHANES 2003–2010. Int J Hyg Environ Health. 2015;218(4):401-6.
22. Wang X, Gao M, Gao J, et al. Extraction of Triclosan and Methyltriclosan in human fluids by in situ ionic liquid morphologic transformation. Journal of Chromatography B. 2018;1092:19-28.
23. Wang H, Zhang A, Wang W, et al. Separation and determination of triclosan and Bisphenol A in water, beverage, and urine samples by dispersive liquid–liquid microextraction combined with capillary zone electrophoresis–UV detection. J AOAC Int. 2013;96(2):459-65.
24. Hesaruiyeh FA, Rajabi S, Motamed-Jahromi M, et al. A pilot study on the association of Lead, 8-Hydroxyguanine, and Malondialdehyde levels in opium addicts’ blood serum with illicit drug use and non-addict persons. Int J Environ Res Public Health. 2022;19(15):9110.
25. Khademi N, Rajabi S, Fararouei M, et al. Environmental exposure to organophosphate pesticides and effects on cognitive functions in elementary school children in a Middle Eastern area. Environ Sci Pollut Res Int. 2023;30(51):111076-91.
26. Chu S, Metcalfe CD. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2007;1164(1-2):212-8.
27. Gatidou G, Thomaidis NS, Stasinakis AS, et al. Simultaneous determination of the endocrine disrupting compounds nonylphenol, nonylphenol ethoxylates, triclosan and bisphenol A in wastewater and sewage sludge by gas chromatography–mass spectrometry. J Chromatogr A. 2007;1138(1-2):32-41.
28. Liu H, Li J, Xia W, et al. Blood pressure changes during pregnancy in relation to urinary paraben, triclosan and benzophenone concentrations: a repeated measures study. Environ Int. 2019;122:185-92.
29. Amin MM, Ebrahimpour K, Parastar S, et al. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547-56.
30. Nasab H, Mirzaee M, Ebrahimpour K, et al. Association of urinary triclosan and methyl-triclosan levels with predictive indicators of cardiovascular disease and obesity in children and adolescents in 2020 (case study: Kerman, Iran). Environmental Health Engineering and Management Journal. 2021;8(3):187-95.
31. Rezaee M, Yamini Y, Faraji M. Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A. 2010;1217(16): 2342-57.
32. Cui Y, Shen N, Wang S, et al. Trace anti-inflammatory β-carboline alkaloid identified in Arenaria kansuensis by two-dimensional chromatography coupled with UniElut C18AEX based solid-phase extraction re-enrichment technology. Journal of Chromatography B. 2017;1068:282-8.
33. Hoogewerff JA, Reimann C, Ueckermann H, et al. Bioavailable 87Sr/86Sr in European soils: a baseline for provenancing studies. Sci Total Environ. 2019;672:1033-44.
34. Asimakopoulos AG, Wang L, Thomaidis NS, et al. A multi-class bioanalytical methodology for the determination of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters, benzophenone-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2014;1324:141-8.
35. Chen M, Zhu P, Xu B, et al. Determination of nine environmental phenols in urine by ultra-high-performance liquid chromatography–tandem mass spectrometry. J Anal Toxicol. 2012;36(9):608-15.
36. Pirard C, Sagot C, Deville M, et al. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ Int. 2012;48:78-83.
37. Gavin QW, Ramage RT, Waldman JM, et al. Development of HPLC-MS/MS method for the simultaneous determination of environmental phenols in human urine. Int J Environ Anal Chem. 2014; 94(2):168-82.
38. Rocha BA, de Oliveira ARM, Barbosa Jr F. A fast and simple air-assisted liquid-liquid microextraction procedure for the simultaneous determination of bisphenols, parabens, benzophenones, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. Talanta. 2018;183:94-101.
39. Spanier AJ, Fausnight T, Camacho TF, et al. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. InAllergy and asthma proceedings; 2014; 35(6):475. OceanSide Publications.
40. Morgan MK, Clifton MS. Exposure to triclosan and bisphenol analogues B, F, P, S and Z in repeated duplicate-diet solid food samples of adults. Toxics. 2021;9(3):47.
41. Calafat AM, Ye X, Wong L-Y, et al. Urinary concentrations of triclosan in the US population: 2003–2004. Environ Health Perspect. 2008;116(3):303-7.
42. Perez AL, Gauthier AM, Ferracini T, et al. The challenge of predicting problematic chemicals using a decision analysis tool: Triclosan as a case study. Integr Environ Assess Manag. 2016;13(1):198-207.
43. Cheng C, Zhou J, Liao J, et al. Investigation on the interactions of contaminant triclosan with human serum albumin: Spectroscopic and molecular docking studies. J Mol Struct. 2024;1295:136737.
Type of Study: Original articles |
Subject:
Environmental Health, Sciences, and Engineering
Received: 2025/06/10 | Accepted: 2025/08/20 | Published: 2025/09/30
Received: 2025/06/10 | Accepted: 2025/08/20 | Published: 2025/09/30
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







