Volume 10, Issue 2 (June 2025)
J Environ Health Sustain Dev 2025, 10(2): 2608-2631 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Wardana L M F, Nurjazuli N, Joko T, Rizaldi M A. Air Pollution Microplastics with the Potential Risk of Lung Disease: A Systematic Review. J Environ Health Sustain Dev 2025; 10 (2) :2608-2631
URL: http://jehsd.ssu.ac.ir/article-1-880-en.html
URL: http://jehsd.ssu.ac.ir/article-1-880-en.html
Public Health Program, Faculty of Health Sciences, Jenderal Soedirman University, Prof. Dr. HR Boenyamin Street No.708, Central Java-53122, Indonesia
Keywords: Microplastics, Particulate Matter, Inhalation Exposure, Air Pollutants, Lung Injury, Toxicity.
Full-Text [PDF 729 kb]
(597 Downloads)
| Abstract (HTML) (715 Views)
Table 1: Search Keywords and Boolean Operators Used In the Literature Search
Table 3: Health Effect Categorization of Airborne Mircoplastics on Respiratory System
Full-Text: (465 Views)
Air Pollution Microplastics with the Potential Risk of Lung Disease:A Systematic Review
Lalu Muhammad Fikri Wardana 1, Nurjazuli 2, Tri Joko 2, Muhammad Addin Rizaldi 3*
1 Department of Environmental Health, Faculty of Public Health, Diponegoro University, Prof. Jacub Rais Street, Central Java-50275, Indonesia.
2Department of Environmental Health, Faculty of Public Health, Diponegoro University, Prof. Jacub Rais Street, Central Java-50275, Indonesia.
3 Public Health Program, Faculty of Health Sciences, Jenderal Soedirman University, Prof. Dr. HR Boenyamin Street No.708, Central Java-53122, Indonesia.
Lalu Muhammad Fikri Wardana 1, Nurjazuli 2, Tri Joko 2, Muhammad Addin Rizaldi 3*
1 Department of Environmental Health, Faculty of Public Health, Diponegoro University, Prof. Jacub Rais Street, Central Java-50275, Indonesia.
2Department of Environmental Health, Faculty of Public Health, Diponegoro University, Prof. Jacub Rais Street, Central Java-50275, Indonesia.
3 Public Health Program, Faculty of Health Sciences, Jenderal Soedirman University, Prof. Dr. HR Boenyamin Street No.708, Central Java-53122, Indonesia.
| A R T I C L E I N F O | ABSTRACT | |
| REVIEW ARTICLE | Introduction: Airborne microplastics (AMPs), due to their small size and widespread dispersal, pose increasing risks to human respiratory health. Detected in both indoor and outdoor environments, AMPs raise concerns over chronic inhalation exposure. Their accumulation in lung tissue may lead to oxidative stress, inflammation, and epithelial barrier dysfunction. This systematic review evaluates the respiratory health effects of AMPs exposure. Materials and Methods: Following PRISMA guidelines, relevant articles were identified through systematic searches in Google Scholar, PubMed, Science Direct, and Springer Link. A total of 20 studies published between 2019 and 2024 were synthesized. Results: AMPs originate from degraded plastics and industrial emissions and can reach alveoli when inhaled. They induce inflammatory responses via oxidative stress and activation of pathways such as NF-κB. Chronic exposure is associated with elevated reactive oxygen species (ROS), mitochondrial dysfunction, and tissue damage, contributing to conditions like pulmonary fibrosis and COPD. AMPs also impair epithelial barriers by disrupting tight junctions and increasing tissue permeability. In vitro and in vivo studies confirm their cytotoxic and inflammatory effects. However, knowledge gaps remain, particularly regarding chronic low-dose exposure and interactions with other pollutants. Conclusion: This review highlights the health risks of AMPs and the need for stricter environmental policies and public education. Findings inform future research and support interventions to mitigate AMPs exposure and protect respiratory health. |
|
Article History: Received: 08 February 2025 Accepted: 20 April 2025 |
||
*Corresponding Author: Muhammad Addin Rizaldi Email: muhammad.rizaldi@unsoed.ac.id Tel: +62 81331917668 |
||
Keywords: Microplastics, Particulate Matter, Inhalation Exposure, Air Pollutants, Lung Injury, Toxicity. |
Citation: Lalu Muhammad Fikri Wardana, Nurjazuli, Tri Joko, et al. Air Pollution Microplastics with the Potential Risk of Lung Disease: A Systematic Review. J Environ Health Sustain Dev. 2025; 10(2): 2608-31.
Introduction
Air pollution has been a human concern for centuries, particularly since the Industrial Revolution. Severe air pollution incidents, such as those in the Meuse Valley (Belgium, 1930) and London (England, 1952), highlight the serious health impacts of localized pollution from industries and power plants1,2. Air pollution is a significant threat to both natural ecosystems and human health worldwide. Urbanization and industrialization are the main causes of air pollution, which is exacerbated by meteorological factors such as high temperatures and low humidity3,4.
Industrial emissions release harmful substances into the air. One such substance released into the atmosphere owing to industrial activities or other processes is microplastics (MPs). MPs, or plastic particles of extremely small size (micro or nano), degrade into smaller particles over their life cycle. For instance, plastic waste exposed to sunlight, wind, or human activities, such as industrial residues or byproducts of motor vehicle combustion, can lead to the formation of MPs5,6.
Airborne Microplastics (AMPs) with a respirable fraction – particles capable of reaching the lower regions of the lungs- are typically smaller than 10 microns, with those less than 5 microns being particularly able to penetrate into the alveolar region. They often exist as microfibers, small fragments, or spherical particles, originating from either primary or secondary plastic products. The main sources of microplastics in the air are human activities, including the fragmentation of larger plastics degraded by natural processes, burning of plastic materials, and use of plastic-based products7. These microplastics are carried by wind, allowing their particles to be distributed across distant regions8.
AMPs can remain suspended in the air for extended periods because of their small size and lightweight nature, making them highly likely to enter the human respiratory tract. Microplastics with small sizes and specific surface properties can adhere to the membranes of lung cells9. Larger particles (> 5 μm) tend to settle in the upper respiratory tract, whereas smaller particles (1–5 μm) and nanoplastics (NPs) can reach the alveoli10.
Microplastics that accumulate in the lungs pose several health risks to humans. Inhaled microplastics can trigger inflammatory responses in lung tissue. More concerningly, owing to their resistance to degradation, microplastics can become trapped within the lung tissue. The long-term accumulation of microplastics in the lungs may lead to conditions such as pulmonary fibrosis or chronic inflammation, thereby increasing the risk of lung cancer10. Another impact of microplastics on the lungs is the increased production of reactive oxygen species (ROS), which contributes to oxidative stress in the lung tissue. Additional risks include lung tissue damage, characterized by thickening of the alveolar walls, damage to lung tissue structures, and increased mucous secretion (Mucin 5AC), indicating a response to injury11.
Although several studies have demonstrated the presence of microplastics in lung tissue, the causal relationship with lung diseases remains unclear and requires further systematic review. This study aimed to compile a systematic review of the existing scientific evidence to evaluate the potential risks of AMPs in lung diseases.
With comprehensive findings, this study is expected to address key challenges related to respirable AMPs, including detection, exposure assessment, and potential health impacts, to support more targeted environmental and public health strategies.
Material and Methods
This study was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Literature searches were performed using several online databases, including Google Scholar, ScienceDirect, PubMed, and SpringerLink. The literature included in the study consisted of publications from December 2019 to December 2024.
To identify relevant literature for the title of this systematic review, keywords were developed using the PICOS framework (participants/population/ problem, interventions, comparisons, outcomes, and study design). Additionally, various synonyms and Medical Subject Headings (MeSH) terms were employed to expand the scope of the search. For instance, synonyms for "microplastic" were searched based on the PICOS framework, including terms such as "NPs,” "plastic microbeads,” "plastic waste,” "plastics,” and "fibrous microplastics.” Similarly, synonyms for study outcomes included terms such as "lung disease,” "lung cancer,” "human exposure,” "interstitial lung disease,” and "pulmonary function.” For air pollution, synonyms included "outdoor air quality,” "air pollution,” "atmospheric pollution,” and "airborne pollutants.”
Subsequently, manual searches were conducted, including a review of the reference lists of previously published articles. The search process used Boolean operators to refine and enhance the results. Boolean operators such as "AND" and "OR" were used. The keywords used for the search are listed in Table 1.
Air pollution has been a human concern for centuries, particularly since the Industrial Revolution. Severe air pollution incidents, such as those in the Meuse Valley (Belgium, 1930) and London (England, 1952), highlight the serious health impacts of localized pollution from industries and power plants1,2. Air pollution is a significant threat to both natural ecosystems and human health worldwide. Urbanization and industrialization are the main causes of air pollution, which is exacerbated by meteorological factors such as high temperatures and low humidity3,4.
Industrial emissions release harmful substances into the air. One such substance released into the atmosphere owing to industrial activities or other processes is microplastics (MPs). MPs, or plastic particles of extremely small size (micro or nano), degrade into smaller particles over their life cycle. For instance, plastic waste exposed to sunlight, wind, or human activities, such as industrial residues or byproducts of motor vehicle combustion, can lead to the formation of MPs5,6.
Airborne Microplastics (AMPs) with a respirable fraction – particles capable of reaching the lower regions of the lungs- are typically smaller than 10 microns, with those less than 5 microns being particularly able to penetrate into the alveolar region. They often exist as microfibers, small fragments, or spherical particles, originating from either primary or secondary plastic products. The main sources of microplastics in the air are human activities, including the fragmentation of larger plastics degraded by natural processes, burning of plastic materials, and use of plastic-based products7. These microplastics are carried by wind, allowing their particles to be distributed across distant regions8.
AMPs can remain suspended in the air for extended periods because of their small size and lightweight nature, making them highly likely to enter the human respiratory tract. Microplastics with small sizes and specific surface properties can adhere to the membranes of lung cells9. Larger particles (> 5 μm) tend to settle in the upper respiratory tract, whereas smaller particles (1–5 μm) and nanoplastics (NPs) can reach the alveoli10.
Microplastics that accumulate in the lungs pose several health risks to humans. Inhaled microplastics can trigger inflammatory responses in lung tissue. More concerningly, owing to their resistance to degradation, microplastics can become trapped within the lung tissue. The long-term accumulation of microplastics in the lungs may lead to conditions such as pulmonary fibrosis or chronic inflammation, thereby increasing the risk of lung cancer10. Another impact of microplastics on the lungs is the increased production of reactive oxygen species (ROS), which contributes to oxidative stress in the lung tissue. Additional risks include lung tissue damage, characterized by thickening of the alveolar walls, damage to lung tissue structures, and increased mucous secretion (Mucin 5AC), indicating a response to injury11.
Although several studies have demonstrated the presence of microplastics in lung tissue, the causal relationship with lung diseases remains unclear and requires further systematic review. This study aimed to compile a systematic review of the existing scientific evidence to evaluate the potential risks of AMPs in lung diseases.
With comprehensive findings, this study is expected to address key challenges related to respirable AMPs, including detection, exposure assessment, and potential health impacts, to support more targeted environmental and public health strategies.
Material and Methods
This study was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Literature searches were performed using several online databases, including Google Scholar, ScienceDirect, PubMed, and SpringerLink. The literature included in the study consisted of publications from December 2019 to December 2024.
To identify relevant literature for the title of this systematic review, keywords were developed using the PICOS framework (participants/population/ problem, interventions, comparisons, outcomes, and study design). Additionally, various synonyms and Medical Subject Headings (MeSH) terms were employed to expand the scope of the search. For instance, synonyms for "microplastic" were searched based on the PICOS framework, including terms such as "NPs,” "plastic microbeads,” "plastic waste,” "plastics,” and "fibrous microplastics.” Similarly, synonyms for study outcomes included terms such as "lung disease,” "lung cancer,” "human exposure,” "interstitial lung disease,” and "pulmonary function.” For air pollution, synonyms included "outdoor air quality,” "air pollution,” "atmospheric pollution,” and "airborne pollutants.”
Subsequently, manual searches were conducted, including a review of the reference lists of previously published articles. The search process used Boolean operators to refine and enhance the results. Boolean operators such as "AND" and "OR" were used. The keywords used for the search are listed in Table 1.
Table 1: Search Keywords and Boolean Operators Used In the Literature Search
| No | Keyword |
| 1 | (Microplastic OR Nanoplastic) AND ("Air Pollution" OR "Airborne Particles") AND ("Inhalation Exposure" OR "Pulmonary Function") |
| 2 | (Microplastic OR "Plastic Fragments") AND ("Urban Air Quality" OR "Particulate Matter") AND ("Respiratory Inflammation" OR "Oxidative Stress") |
| 3 | ("Airborne Microplastics" OR "Plastic Contamination") AND ("Air Quality" OR "PM2.5") AND ("Respiratory Diseases" OR "Pulmonary Disorders") |
| 4 | ("Microplastic Pollution" OR "Plastic Particles") AND ("Outdoor Air Quality") AND ("Pulmonary Dysfunction" OR "Respiratory Toxicity") |
| 5 | (Microplastic OR "Fibrous Microplastic") AND ("Air Contamination") AND ("Inhalation Risk" OR "Breathing Difficulty") |
Inclusion and Exclusion Criteria
The inclusion and exclusion criteria applied in this study were as follows: (1) studies addressing microplastic (MPs) pollution in the air, (2) articles published between 2019 and 2024, (3) research examining the impact of MPs on lung health, (4) original research studies, (5) articles employing experimental, clinical, or observational study designs, (6) studies written in English, and (7) full-text articles accessible for review. The exclusion criteria included: (1) studies discussing MPs in environmental media other than air, (2) articles focusing on MPs effects other than lung health, (3) descriptive or observational studies without toxicity impact measurements of MPs on the lungs, and (4) articles that are not fully accessible.
Data Extraction and Data Synthesis
The data extraction process involved identification, archiving, screening, and eligibility assessment conducted by LMFW, which was reviewed by N, TJ, and AR. The adaptation framework utilized included PICOS, synonyms in keywords, Medical Subject Heading (MeSH) terms, and the number of relevant articles identified. Articles with duplicate titles were removed, and the remaining articles were screened based on their title and abstract. Articles with titles and abstracts that did not meet the inclusion criteria were excluded from the study. A comprehensive review of the full text was conducted for articles that met both the inclusion and exclusion criteria. Information such as citation/author data, study location, study design, participants, study duration, outcomes, findings, limitations, and conclusions were recorded in a Microsoft Excel spreadsheet.
Risk of Bias Evaluation
To minimize the risk of bias in incorporating information into the study, several questions can be posed to ensure the absence of bias, including the following:
The inclusion and exclusion criteria applied in this study were as follows: (1) studies addressing microplastic (MPs) pollution in the air, (2) articles published between 2019 and 2024, (3) research examining the impact of MPs on lung health, (4) original research studies, (5) articles employing experimental, clinical, or observational study designs, (6) studies written in English, and (7) full-text articles accessible for review. The exclusion criteria included: (1) studies discussing MPs in environmental media other than air, (2) articles focusing on MPs effects other than lung health, (3) descriptive or observational studies without toxicity impact measurements of MPs on the lungs, and (4) articles that are not fully accessible.
Data Extraction and Data Synthesis
The data extraction process involved identification, archiving, screening, and eligibility assessment conducted by LMFW, which was reviewed by N, TJ, and AR. The adaptation framework utilized included PICOS, synonyms in keywords, Medical Subject Heading (MeSH) terms, and the number of relevant articles identified. Articles with duplicate titles were removed, and the remaining articles were screened based on their title and abstract. Articles with titles and abstracts that did not meet the inclusion criteria were excluded from the study. A comprehensive review of the full text was conducted for articles that met both the inclusion and exclusion criteria. Information such as citation/author data, study location, study design, participants, study duration, outcomes, findings, limitations, and conclusions were recorded in a Microsoft Excel spreadsheet.
Risk of Bias Evaluation
To minimize the risk of bias in incorporating information into the study, several questions can be posed to ensure the absence of bias, including the following:
- Does the study clearly explain the relationship between airborne MPs exposure and its effects on the lungs?
- Was MPs exposure measured using valid and reliable methods?
- Are the type, size, and concentration of MPs clearly identified
- Were toxicity, oxidative stress, or inflammation measured using reliable instruments?
- Was the study conducted under conditions that reflect real-world MPs exposure?
- Is the statistical analysis employed appropriately to support the conclusions?
- Does the study consider other environmental factors that could influence the research outcomes?
Included and excluded studies
This systematic review included 20 articles obtained through a comprehensive search of several online databases. The details are as follows: Google Scholar yielded 538 articles, Science Direct 349 articles, PubMed 11 articles, and Springer Link 93 articles. A total of 242 duplicate articles were identified and removed, leaving 749 articles recorded and stored for the next stage of the review. After the initial screening by examining the titles and abstracts, the second stage involved a full-text review, resulting in 273 articles that passed. Subsequently, these articles were assessed based on the inclusion and exclusion criteria, leaving 69 articles eligible for a thorough review. From the comprehensive review, 49 articles were eliminated due to relevance and quality considerations, resulting in a final set of 20 articles for the systematic analysis. The details of the PRISMA flow diagram are shown in Figure 1.
Characteristics of included studies
The final analyzed studies were original research. The research designs included clinical, experimental, and observational studies. The sample sizes in each study varied; however, they collectively encompassed the analysis of the impact of MPs on the human alveolar epithelium, bronchoalveolar lavage fluid (BALF), human lung tissue, experimental animal models to assess
human risks, and human bronchial epithelial BEAS-2B cells to evaluate oxidative stress levels.
Result
This study analyzed 20 articles discussing the impact of AMP exposure on human lung health. The findings indicate that MPs, in both micro- and nano-forms, have significant potential adverse effects on the human respiratory system. The detailed findings of this study are presented in Table 2.
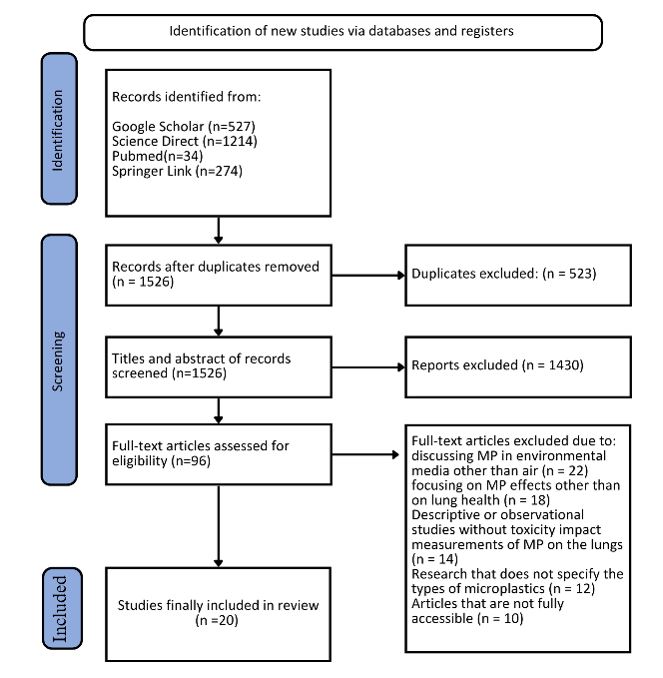
Figure 1: PRISMA Flowchart of Literature Screening and Selection Process
This systematic review included 20 articles obtained through a comprehensive search of several online databases. The details are as follows: Google Scholar yielded 538 articles, Science Direct 349 articles, PubMed 11 articles, and Springer Link 93 articles. A total of 242 duplicate articles were identified and removed, leaving 749 articles recorded and stored for the next stage of the review. After the initial screening by examining the titles and abstracts, the second stage involved a full-text review, resulting in 273 articles that passed. Subsequently, these articles were assessed based on the inclusion and exclusion criteria, leaving 69 articles eligible for a thorough review. From the comprehensive review, 49 articles were eliminated due to relevance and quality considerations, resulting in a final set of 20 articles for the systematic analysis. The details of the PRISMA flow diagram are shown in Figure 1.
Characteristics of included studies
The final analyzed studies were original research. The research designs included clinical, experimental, and observational studies. The sample sizes in each study varied; however, they collectively encompassed the analysis of the impact of MPs on the human alveolar epithelium, bronchoalveolar lavage fluid (BALF), human lung tissue, experimental animal models to assess
human risks, and human bronchial epithelial BEAS-2B cells to evaluate oxidative stress levels.
Result
This study analyzed 20 articles discussing the impact of AMP exposure on human lung health. The findings indicate that MPs, in both micro- and nano-forms, have significant potential adverse effects on the human respiratory system. The detailed findings of this study are presented in Table 2.

Figure 1: PRISMA Flowchart of Literature Screening and Selection Process
Table 2: Study review results
| No | Author (Year) | Objective | Country/region of study | Study Design | Participant | Study Duration | Research Findings | Study Limitation |
| 1 | Xu et al., 201912 | To investigate the internalization and toxicity of NP particles in human lung epithelial cells | China | Experimental in vitro study using confocal microscopy, flow cytometry, RT-PCR, and western blot | Human alveolar epithelial cells (A549) exposed to PS-NPs with diameters of 25 nm and 70 nm at concentrations of 25 µg/mL and 160 µg/mL for 2–8 hours. | 2–8 hours. | PS-NPs 25 was internalized faster than PS-NPs70 through non-specific phagocytosis. PS-NPs triggered apoptosis via the TNF-α pathway, with increased expression of caspase-3, caspase-8, caspase-9, DR5, and cytochrome. The cell cycle showed S-phase arrest with increased expression of cyclin D3 and cyclin E. Inflammatory gene transcription, including IL-6, IL-8, TNF-α, and NF-κB, significantly increased. Greater toxicity was observed with PS-NPs25. | Although in vitro models are ideal for observing cellular effects, the study does not reflect low-dose exposure under real environmental conditions. Long-term effects were not evaluated. |
| 2 | Dong et al., 202013 | To evaluate the effects of polystyrene microplastics (PS-MPs) on human lung health using an in vitro model. The study focused on toxicity, oxidative stress, inflammation, and disruption of the lung epithelial barrier function to understand the molecular mechanisms behind MPs exposure risks. | Taiwan | Experimental in vitro study using human lung epithelial cells (BEAS-2B) cultured in RPMI 1640 medium. Exposure involved PS-MPs at various concentrations (1–1000 µg/cm²) and analysis using methods like DCFH-DA assay for oxidative stress, ELISA for inflammation, and TEER (Trans Epithelial Electrical Resistance) for epithelial barrier integrity. | Human lung epithelial cells (BEAS-2B) cultured under controlled laboratory conditions. No direct human subjects were involved. | Experiments conducted over 24 and 48 hours, with effects evaluated based on key biological parameter changes at each interval. | PS-MPs significantly increased ROS production at high concentrations ( ≥ 10 µg/cm²), triggering oxidative stress that damaged HO-1 protein. Epithelial barrier integrity was disrupted, shown by reduced TEER values and decreased ZO-1 protein expression. At high PS-MPs concentrations, inflammatory cytokine expression (IL-6 and IL-8) increased significantly, indicating activation of inflammatory pathways. Cell viability decreased by 60–70% after 48 hours of exposure to high concentrations of PS-MPs. These findings indicate strong toxic effects of MPs on human lung tissue. | The study used an in vitro model that may not fully represent real human biological responses. Additionally, real environmental conditions such as interactions with other pollutants were not considered. Long-term exposure effects were also not explored. |
| 3 | Yang et al. (2021) 14 | To assess the potential lung toxicity of PS-NPs in the air and understand their underlying mechanisms. | China | In vitro study using human lung epithelial cells and co-culture models. | Human bronchial epithelial cells (BEAS-2B) and human alveolar epithelial cells (HPAEpiC). | Not specified in the document. | PS-NPs significantly reduced cell viability, triggered oxidative stress, inflammatory responses, and apoptosis pathways leading to cell death. PS-NPs also decreased transepithelial electrical resistance by depleting tight junction proteins, potentially leading to tissue damage and lung diseases after prolonged exposure. | This study was conducted in vitro, so the results may not fully represent in vivo conditions. |
| 4 | Lourenço et al., 202115 | To determine the presence of MPs in human lung tissue and analyze their characteristics (size, color, and polymer type) using Raman spectroscopy. | Brazil | Experimental study using human lung tissues obtained from autopsies. | Lung tissues from 20 adult individuals (non-smokers) who had lived in São Paulo for over 10 years, aged 48–94 . | August 2019 – March 2021 | MPs were found in 13 out of 20 lung tissue samples analyzed. A total of 31 particles (87.5% fragments and 12.5% fibers) were identified, with an average size of 3.92 µm for particles and 11.23 µm for fibers. The dominant polymers were polypropylene (35.1%) and polyethylene (24.3%). The study confirmed inhalation as a route of microplastic exposure and concluded that these particles could deposit in human lungs. | Limited sample size (only 20 individuals) and potential particles from other exposure routes (e.g., gastrointestinal translocation). The detection method could not identify nanoparticles. The study did not measure the direct biological impact of MPs on lung tissue. |
| 5 | Lim et al., 202116 | To test the inhalation toxicity of PS-M(N)Ps using a whole-body inhalation system in rats for 14 days, focusing on effects on lung function, inflammation, and molecular expression. | South Korea | Experimental study following modified OECD TG 412 guidelines, including inhalation exposure in a closed test chamber. | 40 Sprague-Dawley rats (20 male, 20 female) divided into control, low, medium, and high exposure groups. | 14 days (6 hours/day, 5 days/week). | Rats showed increased TGF-β and TNF-α expression in lung tissue in a concentration-dependent manner, but no significant changes in physiological lung function were observed at lower concentrations. Respiratory function significantly declined in high-exposure groups for certain parameters (e.g., inspiratory time and respiratory frequency). | Exposure concentrations were higher than typically found in real environmental conditions. The study did not cover long-term effects or multifactorial impact analysis. |
| 6 | Lu et al., 202117 | To understand the effects of microplastic (MPs) exposure on lung physiology under normal conditions and in a mouse model of allergic asthma. The study focused on the influence of MPs on inflammation, immune responses, and genetic expression driving cellular stress and programmed cell death. | China, Germany, Hong Kong SAR | Laboratory experiment with intranasal exposure to synthetic MPs (1–5 µm, density 1.3 g/cc). Mice were exposed intranasally for 24 days (300 µg every three days) with a control group receiving saline. The design included histological analysis, immunofluorescence, ELISA, and transcriptomics. | Female BALB/c mice aged 6–8 weeks, divided into normal and asthma models induced by House Dust Mite (HDM). | 24 days, with intranasal MPs exposure every three days (300 µg per exposure). | MPs caused inflammatory cell infiltration in lung tissue, macrophage aggregation with MPs phagocytosis, increased TNF-α and IgG1 levels in plasma, and expression of genes associated with cellular stress, immune response, and programmed cell death. In the asthma model, MPs exacerbated symptoms such as excessive mucus production, lung tissue inflammation, and airway hyperresponsiveness. Transcriptomic analysis showed dysregulation of genes like HSP90AA1, ITGA4, and TNFRSF13B involved in immunomodulation and apoptosis. | Although the findings are significant, the mouse model only used females, so gender-based differences in responses were not explored. Additionally, the study used MPs of specific sizes (1–5 µm) without evaluating effects of NPs or other size variations. |
| 7 | Goodman et al., 202118 | To evaluate the effects of PS-MPs on the health of human lung epithelial cells, specifically alveolar cells (A549). The study focused on PS-MPs' effects on proliferation, morphology, metabolism, and particle internalization using in vitro models simulating exposure. | United States | Experimental in vitro study using PS-MPs of 1 μm and 10 μm sizes, dissolved in culture medium at concentrations ranging from 0.05–100 μg/mL. Analyses included confocal microscopy, Trypan Blue exclusion, Calcein-AM staining, and Western blot to measure viability, proliferation, and protein expression. | A549 cells, human alveolar epithelial cells, cultured under standard conditions (RPMI-1640 medium with controlled temperature, humidity, and carbon dioxide). | Experiments conducted over 24, 48, 72, and 96 hours, depending on parameters measured (proliferation, metabolism, or morphology). PS-MPs were added at different concentrations to study dose and time effects. | PS-MPs significantly reduced cell proliferation, particularly at high concentrations (50–100 μg/mL). Metabolic activity decreased by up to 45% after 48 hours of exposure, with stronger effects observed with 1 μm PS-MPs compared to 10 μm. Cells exhibited morphological changes, such as filopodia and lamellipodia formation, loss of intercellular adhesion, and PS-MPs internalization near the nucleus. Ki-67 protein expression, a key proliferation marker, decreased by 50% after 72 hours, indicating cell cycle arrest. While overall viability was not significantly affected, oxidative stress induced by PS-MPs was a major concern. | The study used in vitro models that do not fully replicate the complexity of real biological environments. Additionally, PS-MPs were tested in ideal culture media, differing from conditions in human lungs exposed to environmental pollution. Chronic exposure or smaller particles (NPs) were not simulated. |
| 8 | Jenner et al., 202219 | To identify microplastics in human lung tissues using μFTIR. | United Kingdom | Experimental study | 13 human lung tissue samples | Laboratory analysis using μFTIR with human lung tissues | This study identified 39 MPs in lung tissues from 11 out of 13 samples tested, with an average level of 1.42 ± 1.50 MPs/g tissue (before contamination correction) and 0.69 ± 0.84 MPs/g tissue (after contamination correction). The most common MPs were polypropylene (23%), polyethylene terephthalate (18%), and resin (15%). MPs were found in all lung regions (upper, middle, and lower), with the highest concentration in the lower lung (3.12 ± 1.30 MPs/g). The average particle size was 223.10 ± 436.16 μm (length) and 22.21 ± 20.32 μm (width). | Background contamination was tightly controlled using laboratory blank procedures and data correction methods, but the study did not evaluate the direct biological effects of MPs on lung tissues. Donor information, such as smoking status and residential area, was unavailable. |
| 9 | Martínez et al., 202220 | The first study to detect MPs in the human lower respiratory tract using BALF analysis | Spain | Cross-sectional observational study | 44 patients (aged 35–86), consisting of 72.73% men and 27.27% women, with active smokers (52.27%), former smokers (34.09%), and non-smokers (13.64%) | BALF was collected during bronchoscopy using standard techniques. MPs were identified using stereomicroscopy, µ-FTIR, and SEM-EDS. | The average concentration of MPs was 9.18 ± 2.45 items/100 mL BALF. Rayon (40.48%) and polyester (19.05%) were the dominant types of MPs. The concentration of MPs was higher in active smokers (5.26 ± 0.52 items/100 mL) compared to non-smokers (3.14 ± 0.21 items/100 mL). Fibrous MPs accounted for 97.06%, with an average length of 1.73 mm. A significant negative correlation was found between MPs concentration and FEV1/FVC ratio (r = −0.598; p = 0.000), indicating a relationship with impaired lung function. | Procedural blank controls minimized contamination influence. However, the study had limitations in detecting small MPs ( < 20 µm) and did not assess the direct biological impact on lung tissue. |
| 10 | Shi et al., 202221 | To explore the interaction between PS-MPs and lung surfactant (LS) using an in vitro approach. The study focused on changes in LS interfacial properties, mechanisms of ROS formation, and implications for human lung health. | China | Experimental in vitro study using LS extracted from porcine alveolar lavage fluid. Advanced characterization methods such as FTIR, SEM, and UV/Vis spectrophotometry were used. | No direct human or animal participants (laboratory-based study) | Conducted with various PS-MPs concentrations (0–1 mg/L) without explicit time duration | PS-MPs affected LS surface tension, increasing it from 33.77 mN/m to 47.63 mN/m at a PS concentration of 1 mg/L. Phospholipids were more prone to adsorption by PS-MPs than proteins, leading to structural changes in LS membranes. PS-MPs triggered ROS formation (•OH) through mechanisms involving ascorbic acid conversion to dehydroascorbate and hydrogen peroxide (HOOH) production. LS microstructures became unstable, reducing lubrication efficiency and alveolar protection, contributing to lung damage risk. Long-term PS-MPs exposure potentially causes oxidative stress, inflammation, and lung dysfunction. | The study was conducted entirely in vitro, so results may not fully represent real biological conditions. Interaction between PS-MPs and other environmental pollutants was not investigated. The use of porcine LS has limitations in simulating human conditions. |
| 11 | Fan et al., 202222 | To evaluate the toxicity of PS-MPsthrough inhalation in rats, emphasizing molecular mechanisms such as inflammation, lung tissue damage, and the expression of non-coding RNAs (lncRNA and circRNA) associated with inflammatory processes and tissue remodeling. | China | Experimental in vivo study using male Sprague-Dawley rats (aged 6–7 weeks). Rats were exposed to PS-MPs of 100 nm, 500 nm, 1 μm, and 2.5 μm sizes at concentrations of 0.5, 1.0, and 2.0 mg/kg via intratracheal instillation every two days for 14 days. Parameters evaluated included histology, immunofluorescence, ELISA, and RNA sequencing. | 20 Sprague-Dawley rats divided into four groups (control, low dose, medium dose, and high dose), with five rats in each group. Rats were maintained in controlled environmental conditions. | 14 days, with repeated exposure every two days. | Microplastics sized 100 nm exhibited the highest deposition in lung tissues, causing alveolar and bronchial epithelial damage. Inflammatory cytokines such as IL-6, TNF-α, and IL-1β significantly increased in high-dose groups. RNA studies revealed significant changes in 269 circRNAs and 109 lncRNAs. LncRNA XLOC_031479 regulated inflammatory responses, while circRNA 014924 contributed to tissue remodeling. Histological analysis showed interstitial edema and inflammatory cell infiltration in lung tissues. | The study focused on short-term exposure (14 days) and used an animal model. Results cannot be fully extrapolated to humans without further studies. Interactions between PS-MPs and other pollutants were not evaluated. |
| 12 | Zhang et al., 202223 | To investigate the toxicity of polyethylene terephthalate NPs (nano-PET) on human alveolar cells (A549), focusing on internalization, cell viability, oxidative stress, mitochondrial membrane potential, and apoptosis. | China | Experimental in vitro study using nano-PET particles (122–221 nm) exposed to A549 cells at concentrations ranging from 0.10–196.79 µg/mL over 24 hours. | A549 cells, an in vitro model of human alveolar epithelial cells. | 24 hours. | Nano-PET particles were internalized in A549 cells, as detected by confocal microscopy and LC-MS/MS. At low concentrations (0.10–0.98 µg/mL), nano-PET enhanced cell viability. However, at high concentrations (98.40–196.79 µg/mL), cell viability significantly decreased (p < 0.05). Exposure to nano-PET caused increased oxidative stress, demonstrated by elevated ROS levels at concentrations ≥ 49.2 µg/mL. A decline in mitochondrial membrane potential was observed along with ROS elevation. No significant apoptosis occurred at low concentrations, but late-stage apoptosis increased at high doses. | The study was conducted in vitro and over a short duration (24 hours), so long-term effects and biological relevance to humans remain uncertain. |
| 13 | Winkler et al., 202224 | To develop a microplastic fiber (MPF) exposure model using human airway organoids (HAO) and evaluate the effects on morphology, gene expression, and biological responses. The study also explores the integration of fibers into organoid tissues and their impact on airway epithelial repair. | Italy | Experimental in vitro study using HAO developed from lung tissue biopsies of healthy donors. Organoids were exposed to MPFs collected from dryer lint filters and characterized using SEM and ATR-FTIR. Gene expression was analyzed using qRT-PCR, and morphological changes were assessed using confocal microscopy. | HAOcultured in three-dimensional (3D) environments using specialized media to support complex epithelial growth. | 17 days with MPF exposure at concentrations reflecting potential environmental exposure. Measurements were taken at specific intervals to evaluate morphological and gene expression changes. | MPFs affected organoid growth and structure. While no significant barriers to proliferation were observed, MPFs caused polarized cell growth around fibers. The expression of SCGB1A1, a gene critical for club cell function, significantly decreased (p < 0.05), indicating potential impacts on airway function. Microscopic analysis showed MPF integration into organoid tissues, although without triggering significant inflammation or oxidative stress. The findings suggest that MPFs may affect epithelial tissue repair and function over time. | The study used an in vitro model that may not fully replicate the complexity of human lungs. Additionally, MPF concentrations used might not entirely reflect real environmental exposure conditions. Long-term effects were not evaluated. |
| 14 | Halimu et al., 202225 | To understand the toxic mechanisms of NPs (PS-NP) with varying sizes and surface charges on epithelial-mesenchymal transition (EMT) in human lung epithelial cells (A549). The focus was on NOX4 as a mediator of oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress contributing to EMT. | China | Experimental in vitro study using various laboratory techniques to evaluate the toxic effects of NPs, including transwell analysis, ROS measurement, mitochondrial analysis, and western blot. | Human alveolar epithelial type II cells (A549), used to simulate the toxic effects of NPs on the human respiratory system. | Not applicable (cellular model) | PS-NPs induced increased cell migration and EMT marker expression (e.g., MMP2 increased, E-cadherin decreased). PS-NPs triggered oxidative stress, measured by ROS accumulation. Stronger toxic effects were found with smaller PS-NPs and positively charged surface particles. NOX4 played a central role in EMT, mitochondrial dysfunction, and endoplasmic reticulum stress. Mitochondrial dysfunction included changes in membrane potential (Δψm), reduced ATP production, and respiratory chain damage. | The study was based on an in vitro model, which has limitations in replicating the complexity of human biological systems. Environmental effects (e.g., weather factors and particle interactions) were not considered. |
| 15 | Uogintė et al. (2023) 26 | The study aims to detect the presence of MPs NPs in human bronchoalveolar fluid and evaluate their physical and chemical characteristics. | Lithuania | Clinical study using optical microscopy and TEM-EDX analysis. | 10 patients (4 women, 6 men), aged 39-70, from various backgrounds, including smokers and non-smokers from urban and rural areas | - | MPs were found as fragments (84.42%) and fibers (15.65%), with concentrations ranging from 0.11–12.80 particles per 100 mL of bronchoalveolar fluid. NP particles were also detected. No significant relationship was observed between microplastic exposure and environmental, physiological, or clinical factors. The highest MP count was observed in patients suspected of tuberculosis. | Limited sample size, lack of younger population representation, and findings specific to Northern Europe. The TEM-EDX method has limitations in quantifying small nanoparticles. |
| 16 | Luo et al., 202327 | To investigate the molecular mechanisms of lung damage caused by exposure to PS-MPs, with emphasis on the role of circ_kif26b in regulating alveolar epithelial cell senescence through the miR-346-3p/p21 pathway. The study combined in vivo mouse models and in vitro human alveolar epithelial cells to assess the long-term effects of PS-MPs on lung health. | China | In vivo experimental study on male Sprague-Dawley (SD) rats (aged 6–7 weeks) exposed to PS-MPs via inhalation daily for 35 days. In vitro experiments were conducted on MLE12 human alveolar epithelial cells exposed to various concentrations of PS-MPs (0–400 μg/mL) for 48 hours. Exposure in rats was through an inhalation system, while in MLE12 cells, PS-MPs were added to culture media. | 30 male SD rats divided into control and PS-MPs exposure groups (low, medium, high). MLE12 cells used for in vitro analysis | 35 days (in vivo) and 48 hours (in vitro). | PS-MPs induced senescence in alveolar epithelial cells, demonstrated by increased expression of senescence markers such as p21, p16, and p27, along with secretion of the senescence-associated secretory phenotype (SASP), including IL-6, IL-8, and TNF-α. Histopathological analysis showed structural changes in rat lung tissues, including alveolar damage and inflammatory cell infiltration. Reduced epithelial barrier integrity was observed. RNA and proteomic analyses revealed that circ_kif26b acted as a miRNA sponge, binding to miR-346-3p, which regulated p21 expression and triggered cell senescence. PS-MPs also caused mitochondrial membrane potential loss related to increased ROS production. | The study's limitations include the relatively short exposure duration (35 days), which may not fully simulate long-term effects, and the use of male rats, which may not represent the general human population. PS-MPs concentrations used in rats and human cells were higher than typical environmental exposure levels. |
| 17 | Woo et al., 202328 | To investigate the inflammatory mechanisms and lung damage caused by exposure to polypropylene (PP) NPs with a focus on the p38-mediated NF-κB pathway, which is involved in mitochondrial damage | Korea | Experimental: In vivo (male ICR rats), In vitro (human alveolar epithelial cells A549). | 7-week-old male ICR rats, average weight 35.57 g; Human alveolar epithelial cells (A549). | 4 weeks (in vivo), 16 hours (in vitro). | Increased inflammation (cytokines TNF-α, IL-1β, IL-6, MCP-1, CXCL1/KC), ROS production, and mitochondrial damage (membrane depolarization, ATP decrease) were observed in rat lungs and A549 cells. Histopathology showed inflammatory cell infiltration and alveolar hyperplasia. The p38-NF-κB pathway played a significant role in inflammation due to PP exposure. p38 and ROS inhibitors effectively reduced inflammation and cell death. | Exposure doses were higher than those typically found in real-world environments; long-term effects were not evaluated. |
| 18 | Gosselink et al., 202429 | To evaluate the impact of PS PP) MPs on lung health through an in vitro model and to compare the cytotoxic and inflammatory responses of various lung epithelial culture models. | Europe | In vitro study using air-liquid interface (ALI) models with various combinations of lung epithelial cells, including monoculture and co-culture. | A549 cells, EA.hy926 cells, THP-1 cells differentiated into macrophages, human bronchial epithelial cells (PBEC). | 24-30 days for cell differentiation, followed by 24-hour exposure. | PS and PP microplastics caused significant inflammatory responses and cytotoxicity in lung epithelial cells. More complex ALI models exhibited stronger responses to MP exposure compared to simpler models. The study also tested non-plastic nanoparticles as references, including copper(II) oxide (CuO) and titanium dioxide (TiO2). | The study was conducted in vitro, so the results may not fully represent in vivo conditions. Data on the toxicity of microplastics remain limited, requiring further research to understand long-term effects. |
| 19 | Yang et al., 202430 | To investigate the effects of inhaled PS-NPs on lungs using a mouse model, focusing on acute, subacute, and subchronic exposure to identify COPD risks and underlying molecular mechanisms. | China | Laboratory experiment using animal models with oronasal aspiration inhalation towers without anesthesia to minimize stress. | Male C57BL/6 mice (6–8 weeks old) in a controlled environment, exposed to PS-NPs in three doses (16, 40, and 100 μg/day) over three durations (1 week, 1 month, and 3 months). | 1 week (acute), 1 month (subacute), and 3 months (subchronic). | PS-NPs accumulated in the lungs, triggering systemic and local oxidative stress, inflammation, protease-antiprotease imbalance, mitochondrial dysfunction, and endoplasmic reticulum stress. Long-term effects included decreased lung function (EF50 reduction), airway remodeling, and lung fibrosis. Genetic analysis revealed increased expression of COPD-related genes, including MMP-9 (inflammatory marker) and decreased AAT (protease inhibitor). Subcellular mechanisms involved ferroptosis as a key factor in lung injury. | The study was conducted on animal models, so the results do not directly reflect human exposure. There was no direct exploration of human subjects or real environmental conditions. |
| 20 | Roy et al., 202431 | To evaluate the health risks of inhaled MPs and trace metals bound to PM10 in indoor, subway, and outdoor environments. | Seoul, South Korea | Experimental research with air particle analysis and health risk assessment. | No direct human participants; human exposure was simulated based on environmental data. | March 2022 to February 2023. | The highest MPs concentrations were found in subways (four times higher than in outdoor environments) and indoor home environments. MPs deposition per gram of lung tissue: indoor (23.77 MPs/g), subway (1.76 MPs/g), outdoor (2.78 MPs/g). The risk of cancer from heavy metals was higher indoors compared with subways or outdoors. MPs exposure may cause damage to the LS layer and contribute to chronic lung diseases. | No direct biological data from humans. The study used simulation models with fixed assumptions (e.g., lung weight data from Japan). Exposure parameters such as duration and frequency had some uncertainties. |
Discussion
Based on the results of the literature review, several key findings related to the impact of MPs on lung health were identified. These findings include respiratory disturbances, oxidative stress, tissue inflammation, and dysfunction of the epithelial barrier. These findings were categorized through a thorough analysis of the reviewed articles and are explained in Table 3. This table categories the effects in the reviewed studies.
Based on the results of the literature review, several key findings related to the impact of MPs on lung health were identified. These findings include respiratory disturbances, oxidative stress, tissue inflammation, and dysfunction of the epithelial barrier. These findings were categorized through a thorough analysis of the reviewed articles and are explained in Table 3. This table categories the effects in the reviewed studies.
Table 3: Health Effect Categorization of Airborne Mircoplastics on Respiratory System
| No | Category effect | Article number | Percentage |
| 1 | Respiratory disorders | 1, 6, 7, 10, 12, 13, 14 | 35% |
| 2 | Oxidative stress | 2, 3, 5, 7, 9, 11, 15 | 30% |
| 3 | Tissue inflammation | 2, 3, 6, 7, 8, 10, 13, 14, 17 | 50% |
| 4 | Epithelial barrier dysfunction | 2, 3, 5, 7, 10, 12, 13, 15 | 40% |
Respiratory Disorders
The review findings indicate that respiratory disorders are one of the primary effects of airborne MPs exposure. A study identified MP particles in bronchoalveolar fluid at concentrations of up to 12.8 particles per 100 ml. This provides evidence for the presence of MPs in the human respiratory tract. Supported by other studies, airborne MPs and NPs can be deposited in lung tissues and detected in BALF and sputum. These particles have a high deposition potential in the alveoli, posing a risk of triggering respiratory disorders32.
Meanwhile, Martínez et al. (2022) described a reduction in the FEV1/FVC ratio among smokers exposed to MPs. This suggests that MPs have the potential to exacerbate lung function, particularly in individuals with pre-existing risk factors or diseases. Other studies have shown that MPs exposure significantly affects lung function in both smokers and non-smokers. Inhaled MPs, especially small fibers, can settle in the lower respiratory tract, causing inflammation that disrupts gas exchange and reduces lung elasticity. For smokers, this results in compounded effects, whereas for non-smokers, environmental and indoor exposure demonstrates the accumulation of MPs in bronchoalveolar fluid, predominantly consisting of polyester and polyethylene33.
Oxidative stress
Exposure to MPs has been shown to trigger the production of ROS, contributing to oxidative stress in lung tissues. Yang et al. (2021) and Zhang et al. (2022) explained that NPs disrupt mitochondrial function, leading to increased ROS levels and reduced cellular energy capacity. This results in significant mitochondrial dysfunction, which induces apoptosis and cellular damage. Consistent with other studies, MPs exposure promotes ROS production through molecular mechanisms involving direct interactions with cell membranes and mitochondrial damage. MPs act as electron donors, generating free radicals and exacerbating oxidative stress through structural degradation33
According to the findings of Fan et al. (2022), thickening of the alveolar walls and tissue damage indicate that ROS accumulation destructively impacts tissue structure and function, particularly at the cellular level. Chronic MPs exposure may accelerate lung tissue degeneration, increasing the risk of diseases such as emphysema and pulmonary fibrosis.
These findings are supported by other studies showing that ROS enhances mitochondrial fragmentation via DRP1 activation, accelerating mitochondrial dysfunction, loss of mitochondrial membrane potential, and apoptosis34. Additionally, SIRT3 deficiency, a mitochondrial protein responsible for ROS detoxification, exacerbates mitochondrial DNA damage and accelerates lung fibrosis due to oxidative stress. In the context of MPs, uncontrolled ROS elevation can worsen alveolar tissue degeneration, contributing to alveolar wall thickening and the development of lung diseases, such as fibrosis and emphysema35.
In addition to causing direct damage, oxidative stress acts as a mediator of inflammatory pathways. Increased ROS levels can activate signaling pathways involved in immune response regulation, such as NF-κB and proinflammatory cytokines36.
Tissue inflammation
PS-NPs have been shown to significantly trigger inflammatory responses through the cGAS-STING pathway, mediating the release of pro-inflammatory cytokines such as IL-6, MCP-1, and TNF-α. This mechanism induces oxidative stress and disrupts the stability of the alveolar-capillary barrier, allowing particle penetration into the bloodstream and increasing the risk of chronic obstructive pulmonary disease (COPD) due to nanoplastic exposure37. Activation of this pathway also influences the transcription of inflammatory genes, such as NF-κB, contributing to apoptosis and excessive immune responses. The reduction in inflammatory effects following silencing of the STING gene using siRNA confirmed the critical role of this pathway in PS-NPs immunotoxicity38.
Other studies have demonstrated that PP-NPs induce lung tissue inflammation through cellular mechanisms involving the activation of the p38 MAPK and NF-κB pathways. This inflammatory response is characterized by increased levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, as well as inflammatory cell infiltration in the alveoli. Histopathological analyses have revealed alveolar epithelial hyperplasia and foam macrophage accumulation, indicating chronic inflammation and the risk of long-term lung tissue damage39.
Supporting findings have also been reported in the literature. Uogintė et al. (2023) identified MPs and NPs in bronchoalveolar fluid at concentrations of up to 12.8 particles per 100 ml. These particles have the potential to induce oxidative stress and inflammation in the lung tissue. Similarly, Yang et al. (2021) highlighted that PS-NPs can damage epithelial barriers and reduce transepithelial electrical resistance, further exacerbating tissue inflammation.
Epithelial Barrier Dysfunction
According to the review findings, research by Gosselink et al. (2024) and several other studies indicate that MPs, including PS and PP, significantly affect the lung epithelium. This includes epithelial barrier damage caused by disruptions in cellular structures and the infiltration of microplastic particles.
Other studies have shown that exposure to PLA-NPLs in Calu-3 bronchial epithelial models reduces tight junction protein expression by up to 50%. This reduction indicates epithelial barrier instability in the intestinal mucosa. Furthermore, long-term exposure increases epithelial permeability and mucus secretion, making the barrier more vulnerable to infiltration by foreign particles40.
Inhaled microplastics can easily penetrate the alveolar epithelial barrier because of their small size and high surface area-to-volume ratio. This leads to increased barrier permeability and epithelial structural disruption, which, over time, weakens the protective function of the epithelium against harmful environmental exposures41. This condition is exacerbated by the ability of microplastics to interfere with key cellular components, contributing to the overall dysfunction of epithelial barrier function.
Theoretically, MPs and NPs exposure can be analyzed using the epithelial barrier theory framework. This theory suggests that exposure to toxic substances weakens the protective epithelial barriers and increases tissue permeability. Key structures, such as tight junctions, are primary targets for damage, resulting in the loss of epithelial protective function. Small MPs disrupt cellular structures, increasing passive diffusion through paracellular pathways. This allows foreign particles and harmful substances to cross the epithelial barrier, exposing deeper lung tissues to greater risks42.
Conclusion
AMPs, particularly those of respirable size (< 10 µm), can penetrate the lower respiratory tract and trigger oxidative stress, pro-inflammatory responses, epithelial barrier disruption, and overall pulmonary dysfunction. Findings from both in vitro and in vivo studies have demonstrated alterations in the expression of pro-inflammatory cytokines, increased production of ROS, and reduction in epithelial cell integrity. While experimental evidence remains consistent, epidemiological studies in human populations remain limited. Therefore, these findings warrant further investigation through large-scale, population-based research conducted under environmentally realistic exposure scenarios. A more comprehensive assessment is needed to elucidate chronic exposure levels, ambient concentrations of AMPs, and the potential combined effects of AMPs with other pollutants. The potential combined health effects of simultaneous exposure to AMPs and other air pollutants remain poorly understood. This is mainly due to the lack of long-term studies and limited research that reflects real-world environmental exposure. Studies involving human populations are still very limited, making it difficult to fully understand the risks. Therefore, future research should prioritize standardized, long-term, and multidisciplinary studies that assess the effects of chronic exposure and interactions between AMPs and other pollutants.
Abbreviations
AMPs: Airborne Microplastics
MPs: Microplastics
NPs: Nanoplastics
ROS: Reactive Oxygen Species
COPD: Chronic Obstructive Pulmonary Disease
NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells
PS-NPs: Polystyrene Nanoplastics
PS-MPs: Polystyrene Microplastics
PP: Polypropylene
TEER: Transepithelial Electrical Resistance
ELISA: Enzyme-Linked Immunosorbent Assay
BALF: Bronchoalveolar Lavage Fluid
μFTIR: Micro-Fourier Transform Infrared Spectroscopy
SEM-EDS: Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
HDM: House Dust Mite
EMT: Epithelial-Mesenchymal Transition
qRT-PCR: Quantitative Reverse Transcription Polymerase Chain Reaction
ALI: Air-Liquid Interface
lncRNA: Long Non-Coding RNA
circRNA: Circular RNA
SASP: Senescence-Associated Secretory Phenotype
PM10: Particulate Matter smaller than 10 microns
PM2.5: Particulate Matter smaller than 2.5 microns
MMP-9: Matrix Metallopeptidase 9
AAT: Alpha-1 Antitrypsin
ROS-DRP1: Reactive Oxygen Species - Dynamin-related Protein 1 (mitochondrial fission factor)
Acknowledgments
The authors sincerely express profound gratitude to the Shahid Sadoughi University of Medical Sciences for their invaluable support in facilitating the publication of this article.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any funding.
Ethical Considerations
The authors affirm adherence to ethical standards in conducting and reporting this systematic review, including compliance with publication ethics, avoidance of plagiarism, and accurate citation of original works. The study was based solely on previously published data and did not involve human or animal subjects directly. The authors confirm that the work is original, not submitted elsewhere.
Code of Ethics
This article represents an open scientific research study that has not been formally registered as a research project at any university. However, all ethical considerations related to research conduct and reporting have been fully observed and complied with.
Authors' Contributions
All authors contributed equally to the study design, data collection, data analysis, and manuscript preparation
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Brunekreef B. Air pollution and human health: from local to global issues. Procedia Soc Behav Sci. 2010;2(5):6661–9.
2. Rezaali M, Fouladi-Fard R. A narrative summary of air pollution awareness: the recent modeling implications. Journal of Environmental Health and Sustainable Development. 2021;6(1):1175-7.
3. Pekdogan T, Udriștioiu MT, Yildizhan H, et al. From local issues to global impacts: evidence of air pollution for romania and turkey. Sensors. 2024;24(4):1320.
4. Safari Z, Fouladi-Fard R, Vahedian M, et al. Health impact assessment and evaluation of economic costs attributed to PM2.5 air pollution using BenMAP-CE. Int J Biometeorol. 2022;66(9):1891–902.
5. Nematollahi MJ, Keshavarzi B, Mohit F, et al. Microplastic occurrence in urban and industrial soils of Ahvaz metropolis: a city with a sustained record of air pollution. Sci Total Environ. 2022;819:152051.
6. Saeedi R, Khani Jazani R, Khaloo SS, et al. Risk assessment of occupational and public exposures to airborne particulate matter arising from a subway construction site in Tehran, Iran. Air Qual Atmos Health. 2021;14(6):855–62.
7. Ranjdoost F, Abbasi S, Asadi-Ghalhari M, et al. On the nature and sources of microplastics (MPs) and microrubbers (MRs) in urban snow. J Environ Manage. 2024;370:122851.
8. Shao L, Li Y, Jones T, et al. Airborne microplastics: a review of current perspectives and environmental implications. J Clean Prod. 2022;347:131048.
9. Vattanasit U, Kongpran J, Ikeda A. Airborne microplastics: a narrative review of potential effects on the human respiratory system. Sci Total Environ. 2023;904:166745.
10. Prata JC. Airborne microplastics: Consequences to human health?. Environmental pollution. 2018;234:115–26.
11. Wu Y, Yao Y, Bai H, et al. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: the potential protective role of the antioxidant N-acetylcysteine. Sci Total Environ. 2023;855:158851.
12. Xu M, Halimu G, Zhang Q, et al. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019 ;694:133794.
13. Dong CD, Chen CW, Chen YC, et al. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
14. Yang S, Cheng Y, Chen Z, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. 2021;226:112837.
15. Lourenço LFA, Oliveira RC, Júnior GR, et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124.
16. Lim D, Jeong J, Song KS, et al. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262:128330.
17. Lu K, Lai KP, Stoeger T, et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J Hazard Mater. 2021;416:126069.
18. Goodman KE, Hare JT, Khamis ZI, et al. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. 2021;34(4):1069–81.
19. Jenner LC, Rotchell JM, Bennett RT,et al. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831:154907.
20. Martínez CB, Olmos S, Pleiter MG, et al. First evidence of microplastics isolated in European citizens’ lower airway. J Hazard Mater. 2022;438:129439.
21. Shi W, Cao Y, Chai X, et al. Potential health risks of the interaction of microplastics and lung surfactant. J Hazard Mater. 2022;429:128109.
22. Fan Z, Xiao T, Luo H, et al. A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics. Environ Int. 2022;163:107223.
23. Zhang H, Zhang S, Duan Z, et al. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ Int. 2022;162:107177.
24. Winkler AS, Cherubini A, Rusconi F, et al. Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ Int. 2022;163:107200.
25. Halimu G, Zhang Q, Liu L, et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J Hazard Mater. 2022;430:128485.
26. Uogintė I, Vailionytė A, Skapas M, et al. New evidence of the presence of micro- and nanoplastic particles in bronchioalveolar lavage samples of clinical trial subjects. Heliyon. 2023;9(9):e19665.
27. Luo H, Xiao T, Sun X, et al. The regulation of circRNA_kif26b on alveolar epithelial cell senescence via miR-346-3p is involved in microplastics-induced lung injuries. Sci Total Environ. 2023;882:163512.
28. Woo JH, Seo HJ, Lee JY, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023;20(1):2.
29. Gosselink IF, Van Schooten FJ, Drittij MJ, et al. Assessing toxicity of amorphous nanoplastics in airway- and lung epithelial cells using air-liquid interface models. Chemosphere. 2024;368:143702.
30. Yang S, Zhang T, Ge Y, et al. Inhalation exposure to polystyrene nanoplastics induces chronic obstructive pulmonary disease-like lung injury in mice through multi-dimensional assessment. Environmental Pollution. 2024;347: 123633.
31. Roy D, Kim J, Lee M, et al. PM10-bound microplastics and trace metals: a public health insight from the Korean subway and indoor environments. J Hazard Mater. 2024; 477: 135156.
32. Gou Z, Wu H, Li S, et al. Airborne micro- and nanoplastics: emerging causes of respiratory diseases. Part Fibre Toxicol. 2024;21(1):1-22.
33. Sharma N, Kumar V, S. V, et al. Microplastic residues in clinical samples: a retrospection on sources, entry routes, detection methods and human toxicity. Trends Analyt Chem. 2024;173:117618.
34. Liu X, Zhao X, Li X, et al. PM2.5 triggered apoptosis in lung epithelial cells through the mitochondrial apoptotic way mediated by a ROS-DRP1-mitochondrial fission axis. J Hazard Mater. 2020;397:122608.
35. Jablonski RP, Kim S, Cheresh P, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. The FASEB Journal. 2017;31(6):2520–32.
36. Sul OJ, Ra SW. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26(22):6949.
37. Yang S, Zhang T, Ge Y, et al. Sentinel supervised lung-on-a-chip: a new environmental toxicology platform for nanoplastic-induced lung injury. J Hazard Mater. 2023;458:131962.
38. Xuan L, Wang Y, Qu C, et al. Exposure to polystyrene nanoplastics induces abnormal activation of innate immunity via the cGAS-STING pathway. Ecotoxicol Environ Saf. 2024;275:116255.
39. Woo JH, Seo HJ, Lee JY, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023;20(1):2.
40. Rodríguez AG, Gutiérrez J, Villacorta A, et al. Polylactic acid nanoplastics (PLA-NPLs) induce adverse effects on an in vitro model of the human lung epithelium: the Calu-3 air-liquid interface (ALI) barrier. J Hazard Mater. 2024;475:134900.
41. Choudhury A, Simnani FZ, Singh D, et al. Atmospheric microplastic and nanoplastic: the toxicological paradigm on the cellular system. Ecotoxicol Environ Saf. 2023;259:115018.
42. Losol P, Sokolowska M, Hwang YK, et al. Epithelial Barrier theory: the role of exposome, microbiome, and barrier function in allergic diseases. Allergy Asthma Immunol Res. 2023;15(6):705.
The review findings indicate that respiratory disorders are one of the primary effects of airborne MPs exposure. A study identified MP particles in bronchoalveolar fluid at concentrations of up to 12.8 particles per 100 ml. This provides evidence for the presence of MPs in the human respiratory tract. Supported by other studies, airborne MPs and NPs can be deposited in lung tissues and detected in BALF and sputum. These particles have a high deposition potential in the alveoli, posing a risk of triggering respiratory disorders32.
Meanwhile, Martínez et al. (2022) described a reduction in the FEV1/FVC ratio among smokers exposed to MPs. This suggests that MPs have the potential to exacerbate lung function, particularly in individuals with pre-existing risk factors or diseases. Other studies have shown that MPs exposure significantly affects lung function in both smokers and non-smokers. Inhaled MPs, especially small fibers, can settle in the lower respiratory tract, causing inflammation that disrupts gas exchange and reduces lung elasticity. For smokers, this results in compounded effects, whereas for non-smokers, environmental and indoor exposure demonstrates the accumulation of MPs in bronchoalveolar fluid, predominantly consisting of polyester and polyethylene33.
Oxidative stress
Exposure to MPs has been shown to trigger the production of ROS, contributing to oxidative stress in lung tissues. Yang et al. (2021) and Zhang et al. (2022) explained that NPs disrupt mitochondrial function, leading to increased ROS levels and reduced cellular energy capacity. This results in significant mitochondrial dysfunction, which induces apoptosis and cellular damage. Consistent with other studies, MPs exposure promotes ROS production through molecular mechanisms involving direct interactions with cell membranes and mitochondrial damage. MPs act as electron donors, generating free radicals and exacerbating oxidative stress through structural degradation33
According to the findings of Fan et al. (2022), thickening of the alveolar walls and tissue damage indicate that ROS accumulation destructively impacts tissue structure and function, particularly at the cellular level. Chronic MPs exposure may accelerate lung tissue degeneration, increasing the risk of diseases such as emphysema and pulmonary fibrosis.
These findings are supported by other studies showing that ROS enhances mitochondrial fragmentation via DRP1 activation, accelerating mitochondrial dysfunction, loss of mitochondrial membrane potential, and apoptosis34. Additionally, SIRT3 deficiency, a mitochondrial protein responsible for ROS detoxification, exacerbates mitochondrial DNA damage and accelerates lung fibrosis due to oxidative stress. In the context of MPs, uncontrolled ROS elevation can worsen alveolar tissue degeneration, contributing to alveolar wall thickening and the development of lung diseases, such as fibrosis and emphysema35.
In addition to causing direct damage, oxidative stress acts as a mediator of inflammatory pathways. Increased ROS levels can activate signaling pathways involved in immune response regulation, such as NF-κB and proinflammatory cytokines36.
Tissue inflammation
PS-NPs have been shown to significantly trigger inflammatory responses through the cGAS-STING pathway, mediating the release of pro-inflammatory cytokines such as IL-6, MCP-1, and TNF-α. This mechanism induces oxidative stress and disrupts the stability of the alveolar-capillary barrier, allowing particle penetration into the bloodstream and increasing the risk of chronic obstructive pulmonary disease (COPD) due to nanoplastic exposure37. Activation of this pathway also influences the transcription of inflammatory genes, such as NF-κB, contributing to apoptosis and excessive immune responses. The reduction in inflammatory effects following silencing of the STING gene using siRNA confirmed the critical role of this pathway in PS-NPs immunotoxicity38.
Other studies have demonstrated that PP-NPs induce lung tissue inflammation through cellular mechanisms involving the activation of the p38 MAPK and NF-κB pathways. This inflammatory response is characterized by increased levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, as well as inflammatory cell infiltration in the alveoli. Histopathological analyses have revealed alveolar epithelial hyperplasia and foam macrophage accumulation, indicating chronic inflammation and the risk of long-term lung tissue damage39.
Supporting findings have also been reported in the literature. Uogintė et al. (2023) identified MPs and NPs in bronchoalveolar fluid at concentrations of up to 12.8 particles per 100 ml. These particles have the potential to induce oxidative stress and inflammation in the lung tissue. Similarly, Yang et al. (2021) highlighted that PS-NPs can damage epithelial barriers and reduce transepithelial electrical resistance, further exacerbating tissue inflammation.
Epithelial Barrier Dysfunction
According to the review findings, research by Gosselink et al. (2024) and several other studies indicate that MPs, including PS and PP, significantly affect the lung epithelium. This includes epithelial barrier damage caused by disruptions in cellular structures and the infiltration of microplastic particles.
Other studies have shown that exposure to PLA-NPLs in Calu-3 bronchial epithelial models reduces tight junction protein expression by up to 50%. This reduction indicates epithelial barrier instability in the intestinal mucosa. Furthermore, long-term exposure increases epithelial permeability and mucus secretion, making the barrier more vulnerable to infiltration by foreign particles40.
Inhaled microplastics can easily penetrate the alveolar epithelial barrier because of their small size and high surface area-to-volume ratio. This leads to increased barrier permeability and epithelial structural disruption, which, over time, weakens the protective function of the epithelium against harmful environmental exposures41. This condition is exacerbated by the ability of microplastics to interfere with key cellular components, contributing to the overall dysfunction of epithelial barrier function.
Theoretically, MPs and NPs exposure can be analyzed using the epithelial barrier theory framework. This theory suggests that exposure to toxic substances weakens the protective epithelial barriers and increases tissue permeability. Key structures, such as tight junctions, are primary targets for damage, resulting in the loss of epithelial protective function. Small MPs disrupt cellular structures, increasing passive diffusion through paracellular pathways. This allows foreign particles and harmful substances to cross the epithelial barrier, exposing deeper lung tissues to greater risks42.
Conclusion
AMPs, particularly those of respirable size (< 10 µm), can penetrate the lower respiratory tract and trigger oxidative stress, pro-inflammatory responses, epithelial barrier disruption, and overall pulmonary dysfunction. Findings from both in vitro and in vivo studies have demonstrated alterations in the expression of pro-inflammatory cytokines, increased production of ROS, and reduction in epithelial cell integrity. While experimental evidence remains consistent, epidemiological studies in human populations remain limited. Therefore, these findings warrant further investigation through large-scale, population-based research conducted under environmentally realistic exposure scenarios. A more comprehensive assessment is needed to elucidate chronic exposure levels, ambient concentrations of AMPs, and the potential combined effects of AMPs with other pollutants. The potential combined health effects of simultaneous exposure to AMPs and other air pollutants remain poorly understood. This is mainly due to the lack of long-term studies and limited research that reflects real-world environmental exposure. Studies involving human populations are still very limited, making it difficult to fully understand the risks. Therefore, future research should prioritize standardized, long-term, and multidisciplinary studies that assess the effects of chronic exposure and interactions between AMPs and other pollutants.
Abbreviations
AMPs: Airborne Microplastics
MPs: Microplastics
NPs: Nanoplastics
ROS: Reactive Oxygen Species
COPD: Chronic Obstructive Pulmonary Disease
NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells
PS-NPs: Polystyrene Nanoplastics
PS-MPs: Polystyrene Microplastics
PP: Polypropylene
TEER: Transepithelial Electrical Resistance
ELISA: Enzyme-Linked Immunosorbent Assay
BALF: Bronchoalveolar Lavage Fluid
μFTIR: Micro-Fourier Transform Infrared Spectroscopy
SEM-EDS: Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
HDM: House Dust Mite
EMT: Epithelial-Mesenchymal Transition
qRT-PCR: Quantitative Reverse Transcription Polymerase Chain Reaction
ALI: Air-Liquid Interface
lncRNA: Long Non-Coding RNA
circRNA: Circular RNA
SASP: Senescence-Associated Secretory Phenotype
PM10: Particulate Matter smaller than 10 microns
PM2.5: Particulate Matter smaller than 2.5 microns
MMP-9: Matrix Metallopeptidase 9
AAT: Alpha-1 Antitrypsin
ROS-DRP1: Reactive Oxygen Species - Dynamin-related Protein 1 (mitochondrial fission factor)
Acknowledgments
The authors sincerely express profound gratitude to the Shahid Sadoughi University of Medical Sciences for their invaluable support in facilitating the publication of this article.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any funding.
Ethical Considerations
The authors affirm adherence to ethical standards in conducting and reporting this systematic review, including compliance with publication ethics, avoidance of plagiarism, and accurate citation of original works. The study was based solely on previously published data and did not involve human or animal subjects directly. The authors confirm that the work is original, not submitted elsewhere.
Code of Ethics
This article represents an open scientific research study that has not been formally registered as a research project at any university. However, all ethical considerations related to research conduct and reporting have been fully observed and complied with.
Authors' Contributions
All authors contributed equally to the study design, data collection, data analysis, and manuscript preparation
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Brunekreef B. Air pollution and human health: from local to global issues. Procedia Soc Behav Sci. 2010;2(5):6661–9.
2. Rezaali M, Fouladi-Fard R. A narrative summary of air pollution awareness: the recent modeling implications. Journal of Environmental Health and Sustainable Development. 2021;6(1):1175-7.
3. Pekdogan T, Udriștioiu MT, Yildizhan H, et al. From local issues to global impacts: evidence of air pollution for romania and turkey. Sensors. 2024;24(4):1320.
4. Safari Z, Fouladi-Fard R, Vahedian M, et al. Health impact assessment and evaluation of economic costs attributed to PM2.5 air pollution using BenMAP-CE. Int J Biometeorol. 2022;66(9):1891–902.
5. Nematollahi MJ, Keshavarzi B, Mohit F, et al. Microplastic occurrence in urban and industrial soils of Ahvaz metropolis: a city with a sustained record of air pollution. Sci Total Environ. 2022;819:152051.
6. Saeedi R, Khani Jazani R, Khaloo SS, et al. Risk assessment of occupational and public exposures to airborne particulate matter arising from a subway construction site in Tehran, Iran. Air Qual Atmos Health. 2021;14(6):855–62.
7. Ranjdoost F, Abbasi S, Asadi-Ghalhari M, et al. On the nature and sources of microplastics (MPs) and microrubbers (MRs) in urban snow. J Environ Manage. 2024;370:122851.
8. Shao L, Li Y, Jones T, et al. Airborne microplastics: a review of current perspectives and environmental implications. J Clean Prod. 2022;347:131048.
9. Vattanasit U, Kongpran J, Ikeda A. Airborne microplastics: a narrative review of potential effects on the human respiratory system. Sci Total Environ. 2023;904:166745.
10. Prata JC. Airborne microplastics: Consequences to human health?. Environmental pollution. 2018;234:115–26.
11. Wu Y, Yao Y, Bai H, et al. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: the potential protective role of the antioxidant N-acetylcysteine. Sci Total Environ. 2023;855:158851.
12. Xu M, Halimu G, Zhang Q, et al. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019 ;694:133794.
13. Dong CD, Chen CW, Chen YC, et al. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
14. Yang S, Cheng Y, Chen Z, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. 2021;226:112837.
15. Lourenço LFA, Oliveira RC, Júnior GR, et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124.
16. Lim D, Jeong J, Song KS, et al. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262:128330.
17. Lu K, Lai KP, Stoeger T, et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J Hazard Mater. 2021;416:126069.
18. Goodman KE, Hare JT, Khamis ZI, et al. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. 2021;34(4):1069–81.
19. Jenner LC, Rotchell JM, Bennett RT,et al. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831:154907.
20. Martínez CB, Olmos S, Pleiter MG, et al. First evidence of microplastics isolated in European citizens’ lower airway. J Hazard Mater. 2022;438:129439.
21. Shi W, Cao Y, Chai X, et al. Potential health risks of the interaction of microplastics and lung surfactant. J Hazard Mater. 2022;429:128109.
22. Fan Z, Xiao T, Luo H, et al. A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics. Environ Int. 2022;163:107223.
23. Zhang H, Zhang S, Duan Z, et al. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ Int. 2022;162:107177.
24. Winkler AS, Cherubini A, Rusconi F, et al. Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ Int. 2022;163:107200.
25. Halimu G, Zhang Q, Liu L, et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J Hazard Mater. 2022;430:128485.
26. Uogintė I, Vailionytė A, Skapas M, et al. New evidence of the presence of micro- and nanoplastic particles in bronchioalveolar lavage samples of clinical trial subjects. Heliyon. 2023;9(9):e19665.
27. Luo H, Xiao T, Sun X, et al. The regulation of circRNA_kif26b on alveolar epithelial cell senescence via miR-346-3p is involved in microplastics-induced lung injuries. Sci Total Environ. 2023;882:163512.
28. Woo JH, Seo HJ, Lee JY, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023;20(1):2.
29. Gosselink IF, Van Schooten FJ, Drittij MJ, et al. Assessing toxicity of amorphous nanoplastics in airway- and lung epithelial cells using air-liquid interface models. Chemosphere. 2024;368:143702.
30. Yang S, Zhang T, Ge Y, et al. Inhalation exposure to polystyrene nanoplastics induces chronic obstructive pulmonary disease-like lung injury in mice through multi-dimensional assessment. Environmental Pollution. 2024;347: 123633.
31. Roy D, Kim J, Lee M, et al. PM10-bound microplastics and trace metals: a public health insight from the Korean subway and indoor environments. J Hazard Mater. 2024; 477: 135156.
32. Gou Z, Wu H, Li S, et al. Airborne micro- and nanoplastics: emerging causes of respiratory diseases. Part Fibre Toxicol. 2024;21(1):1-22.
33. Sharma N, Kumar V, S. V, et al. Microplastic residues in clinical samples: a retrospection on sources, entry routes, detection methods and human toxicity. Trends Analyt Chem. 2024;173:117618.
34. Liu X, Zhao X, Li X, et al. PM2.5 triggered apoptosis in lung epithelial cells through the mitochondrial apoptotic way mediated by a ROS-DRP1-mitochondrial fission axis. J Hazard Mater. 2020;397:122608.
35. Jablonski RP, Kim S, Cheresh P, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. The FASEB Journal. 2017;31(6):2520–32.
36. Sul OJ, Ra SW. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26(22):6949.
37. Yang S, Zhang T, Ge Y, et al. Sentinel supervised lung-on-a-chip: a new environmental toxicology platform for nanoplastic-induced lung injury. J Hazard Mater. 2023;458:131962.
38. Xuan L, Wang Y, Qu C, et al. Exposure to polystyrene nanoplastics induces abnormal activation of innate immunity via the cGAS-STING pathway. Ecotoxicol Environ Saf. 2024;275:116255.
39. Woo JH, Seo HJ, Lee JY, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023;20(1):2.
40. Rodríguez AG, Gutiérrez J, Villacorta A, et al. Polylactic acid nanoplastics (PLA-NPLs) induce adverse effects on an in vitro model of the human lung epithelium: the Calu-3 air-liquid interface (ALI) barrier. J Hazard Mater. 2024;475:134900.
41. Choudhury A, Simnani FZ, Singh D, et al. Atmospheric microplastic and nanoplastic: the toxicological paradigm on the cellular system. Ecotoxicol Environ Saf. 2023;259:115018.
42. Losol P, Sokolowska M, Hwang YK, et al. Epithelial Barrier theory: the role of exposome, microbiome, and barrier function in allergic diseases. Allergy Asthma Immunol Res. 2023;15(6):705.
Type of Study: Systematic Review |
Subject:
Environmental pollution
Received: 2025/02/8 | Accepted: 2025/04/20 | Published: 2025/06/29
Received: 2025/02/8 | Accepted: 2025/04/20 | Published: 2025/06/29
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







