Volume 10, Issue 1 (March 2025)
J Environ Health Sustain Dev 2025, 10(1): 2499-2520 |
Back to browse issues page
Ethics code: NA
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tushir R, Das S, Chauhan A, Gurjar S, Bhanwala L, Patnaik S et al . Exponential Global Health Hazards of Pharmaceutical Pollution and Its Preventive Measures: A Comprehensive Review. J Environ Health Sustain Dev 2025; 10 (1) :2499-2520
URL: http://jehsd.ssu.ac.ir/article-1-843-en.html
URL: http://jehsd.ssu.ac.ir/article-1-843-en.html
Renu Tushir 
 , Sanjita Das *
, Sanjita Das * 

 , Ajesh Chauhan
, Ajesh Chauhan 
 , Saurav Gurjar
, Saurav Gurjar 
 , Lakshay Bhanwala
, Lakshay Bhanwala 
 , Sneha Patnaik
, Sneha Patnaik 

 , Anushka Jain
, Anushka Jain 



 , Sanjita Das *
, Sanjita Das * 

 , Ajesh Chauhan
, Ajesh Chauhan 
 , Saurav Gurjar
, Saurav Gurjar 
 , Lakshay Bhanwala
, Lakshay Bhanwala 
 , Sneha Patnaik
, Sneha Patnaik 

 , Anushka Jain
, Anushka Jain 


Department of Pharmacy, SMAS, Galgotias University, Plot No. 2, Sector 17-A, Yamuna Expressway, Greater Noida-201310, Uttar Pradesh, India
Keywords: Waste Management, Environment, Biological Products, Environmental Pollution, Antibiotics, Government Agencies.
Full-Text [PDF 999 kb]
(126 Downloads)
| Abstract (HTML) (371 Views)
.PNG)
Figure 1: Estimated research publications on pharmaceutical pollution and its management
The data were obtained from publications published in different years on pharmaceutical pollution. We focused on recent journals that provide more information. This shows that people are becoming concerned about pharmaceutical pollution and want it to be prevented and investigated.
Materials and Methods
The review was prepared by ensuring that the search criteria for pharmaceutical pollution are critical for ensuring the rigor and relevance of the included literature. A systematic search for relevant literature was performed across multiple reputable databases to gather comprehensive and current information on the management of pharmaceutical pollution. The resources used to obtain appropriate information were PubMed, Google Scholar, Scopus, and Web of Science. A careful assessment was carried out to obtain appropriate information about the global issue of pharmaceutical pollution.
Pharmaceuticals affecting the ecosystem
Determining the impact of synthetic and semi-synthetic pharmaceutical formulations on ecosystems is not easy. Currently, it has become evident that categories of drugs, including antifungal, antibacterial, xeno–estrogens, and anti-parasites, are ecologically toxic and pose environmental threats. Between 1996 and 2007, a significant decline occurred in the vulture population, with millions affected by exposure to anti-inflammatory medicines, resulting in near extinction8,9. Due to the necessity of these treatments for alleviating pain and fever in cattle and the disposal of numerous deceased calves for vulture feeding, certain animals were subjected to elevated doses of diclofenac10,11. A significant percentage of birds died due to severe renal failure and abdominal gout. Vultures from the genus Gyps are significantly susceptible to diclofenac, highlighting the detrimental effects of these pharmaceuticals on avian populations, ultimately jeopardizing three Gyps species in Asia12. Physicians recommend against the concomitant use of ibuprofen and beta-blockers; however, this does not apply to marine fish, which are significantly exposed to various combinations of medications and pollutants, as illustrated in Figure 2. Certain studies have demonstrated the feminizing effects of estrogen, including bisphenol-A, present in contraceptive pills on male fish, which adversely impacts the endocrine system13. River pollution is a major issue affecting the entire ecosystem, including the Yamuna River, which is affected by industrial waste. The worst effect was observed in the Okhla and Nizamuddin bridges, making it the seventh worst-affected river with the highest biochemical oxygen demand. However, certain stretches of the river are free from these pollution impacts, such as the Yamuna River originating from the Tajewala Barrage 14,15. According to reports, the top five polluted rivers in India are as follows:
Ganga River>Yamuna>Brahmaputra>Damodar> Bagmati 16.
.PNG)
Figure 2: Illustration of the transportation of pharmaceuticals used by humans back to humans through a series chain 13.
.PNG)
Figure 3: Classification of Hazardous waste chemicals: This figure explains the categorization of different prominent biohazardous pharmaceuticals, and an attempt has been made to segregate them into different specific classes 78.
.PNG)
Figure 4: Worldwide estimated waste production data: This demonstrates that the southern region of Africa is creating pharmaceutical pollution, which might be due to less concern about its harmful effects on the environment. It also shows that along with India, China, North, and South America are also playing an important role in adding pollutants to the environment 70,71.
Full-Text: (168 Views)
Exponential Global Health Hazards of Pharmaceutical Pollution and Its Preventive Measures: A Comprehensive Review
Renu Tushir 1, Sanjita Das 1*, Ajesh Chauhan 2, Saurav Gurjar 3, Lakshay Bhanwala 4,
Sneha Patnaik 5, Anushka Jain 1
1 Department of Pharmacy, SMAS, Galgotias University, Plot No. 2, Sector 17-A, Yamuna Expressway, Greater Noida-201310, Uttar Pradesh, India.
2Hindu College of Pharmacy, PT. BD. Sharma University, Rohtak, India.
3 Department of Cardiovascular Technology, Galgotias University, Greater Noida, Uttar Pradesh-203201, India.
4 RSM College of Pharmacy, PT. BD. Sharma University, Panipat-132103, India.
5 Department of Physiotherapy, Jamia Hamdard University, Hamdard Nagar, New Delhi, India.
Renu Tushir 1, Sanjita Das 1*, Ajesh Chauhan 2, Saurav Gurjar 3, Lakshay Bhanwala 4,
Sneha Patnaik 5, Anushka Jain 1
1 Department of Pharmacy, SMAS, Galgotias University, Plot No. 2, Sector 17-A, Yamuna Expressway, Greater Noida-201310, Uttar Pradesh, India.
2Hindu College of Pharmacy, PT. BD. Sharma University, Rohtak, India.
3 Department of Cardiovascular Technology, Galgotias University, Greater Noida, Uttar Pradesh-203201, India.
4 RSM College of Pharmacy, PT. BD. Sharma University, Panipat-132103, India.
5 Department of Physiotherapy, Jamia Hamdard University, Hamdard Nagar, New Delhi, India.
| A R T I C L E I N F O | GRAPHICAL ABSTRACT | |
| REVIEW ARTICLE |
Introduction: Antimicrobial agents from different categories like antibiotics, anti-parasitic agents, and other agents such as Xeno (estrogens) can exert some intimidating effects on the environment that can be ecotoxicological as well. Disposal of chemicals in the form of drugs in various water bodies such as lakes, ponds, rivers, and commonly used drinking water might cause potential health damage to human, animal, and aquatic life via environmental pollution. Thus, production of such waste requires proper disposal and supervision, which might be a hurdle for the medical personnel, city administrators, workers working in the industry involved in recycling, and planners of policies.
Materials and Methods: The review is taken the help of resources for getting appropriate information are PubMed, Google Scholar, Scopus, Web of Science very carefully. The period of time for article search in databases varied from 2001-2023. The articles between this time range were included. Results: In the present study, an effort was made to pinnacle sources, categories, and information regarding various drugs and regulatory bodies across the globe to control these functions. To overcome the pharmaceutical pollution issues, functions, categories of wastes, and strategies to resolve were elaborated. Conclusion: The study highlighted the crucial function of pharmacists in reducing pharmaceutical waste and evaluated current water management policies, emphasizing the necessity for cohesive efforts to diminish pollution. Moreover, an examination of existing regulatory frameworks revealed inadequacies and provided suggestions for improved monitoring and enforcement. This comprehensive report aimed to offer practical recommendations for the advancement of sustainable pharmaceutical waste management techniques, aiding in the protection of world health and the environment. Highlights:
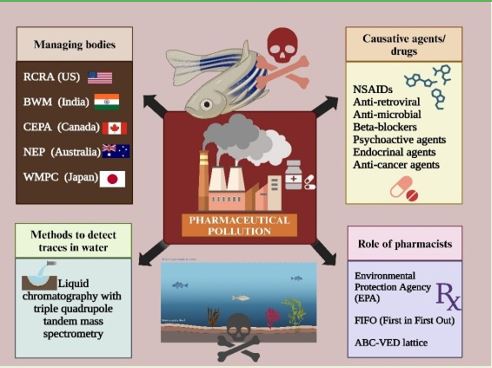 |
|
Article History: Received: 16 November 2024 Accepted: 20 January 2025 |
||
*Corresponding Author: Sanjita Das Email: sanjita8@yahoo.co.in Tel: +91 9818223359 |
||
Keywords: Waste Management, Environment, Biological Products, Environmental Pollution, Antibiotics, Government Agencies. |
Citation: Tushir R, Das S, Chauhan A, et al. Exponential Global Health Hazards of Pharmaceutical Pollution and Its Preventive Measures: A Comprehensive Review. J Environ Health Sustain Dev. 2025; 10(1): 2499-520.
Introduction
Approximately 3000 compounds are used as pharmaceuticals, including analgesics, antibiotics, anti-inflammatory agents, anti-fungal agents, and anti-viral agents, leading to the generation of hundreds of tons of waste in the environment1,2. Attention has been drawn to the upcoming water-soluble and pharmacologically active environmental pollutants. Due to the major health and ecotoxicological implications of pharmaceutical and personal care product (PPCP) water pollution, organic micropollutants and pharmaceutically active substances are a growing concern worldwide. Due to limitless production, uncontrolled consumption, and improper disposal, there has been an increased spread of these substances in the environment. As far as closing the gap between developed and pharmerging markets bearing countries is concerned, Saudi Arabia is the first among the rest of the countries that will close the gap by 10% or more Turkey, Algeria, Egypt, Columbia, Bangladesh, and Brazil. During their production, use, and removal, active pharmaceutical ingredients (APIs), like other synthetic fixings, are delivered into the climate. Drugs (principally restorative items, yet additionally other individual consideration items) may be seen as natural poisons attributed to their usage in both human and veterinary medicine3. Moreover, 559 unique medication trimmings were found in biological territories, such as surface water, groundwater, and soil. Approximately 3000 potent pharmaceutical derivatives have been approved for sale in the EU, with annual per capita therapeutic applications ranging from 50 to 150 grams per capita annually4. Activities involving sewage treatment, surface runoff, soil sifting, and manure application frequently result in the release of pharmaceuticals and metabolites into the marine ecosystem via urine and faeces5. Aquafarming requires veterinary medications, leading to their direct release into the environment. Topical treatments are indirectly related to runoff, manure, and animal feces from fields, which are the primary sources 6. A worldwide survey showed that more than 600 distinctive APIs have been recognized in the climate, sometimes at levels that represent a high danger to the climate7.
Extensive research is being conducted to address harmful pharmaceutical pollutants, which have become a significant concern because of the growing risk they pose to society. Consequently, the number of research publications on this topic has steadily increased, as shown in Figure 1. This study aims to fill the existing gaps in understanding common pharmaceutical pollution and explore the critical role of pharmacists in mitigating these issues. It also examines various regulatory agencies involved in pharmaceutical waste management, offering a comparative analysis of the schedules and guidelines of different countries. Previous studies have often overlooked the comprehensive understanding of the environmental impact of pharmaceutical pollution and the collaborative efforts needed across sectors. This study addresses these gaps by providing a more holistic view of pharmaceutical pollution management and the specific actions required from key regulatory authorities in India.
Inclusion/exclusion criteria
The inclusion and exclusion criteria were designed to ensure the selection of relevant, high-quality, and impactful research. The inclusion criteria focused on peer-reviewed articles, systematic reviews, meta-analyses, and original research that discussed pharmaceutical pollution sources, global health hazards, and preventive strategies, including regulatory measures and the role of pharmacists. The exclusion criteria eliminated non-peer-reviewed or opinion-based articles, studies lacking primary data or methodological rigor, and those limited to unrelated pollutants or isolated local effects without global relevance. This approach ensures a comprehensive review of the global health hazards of pharmaceutical pollution and its mitigation strategies.
Approximately 3000 compounds are used as pharmaceuticals, including analgesics, antibiotics, anti-inflammatory agents, anti-fungal agents, and anti-viral agents, leading to the generation of hundreds of tons of waste in the environment1,2. Attention has been drawn to the upcoming water-soluble and pharmacologically active environmental pollutants. Due to the major health and ecotoxicological implications of pharmaceutical and personal care product (PPCP) water pollution, organic micropollutants and pharmaceutically active substances are a growing concern worldwide. Due to limitless production, uncontrolled consumption, and improper disposal, there has been an increased spread of these substances in the environment. As far as closing the gap between developed and pharmerging markets bearing countries is concerned, Saudi Arabia is the first among the rest of the countries that will close the gap by 10% or more Turkey, Algeria, Egypt, Columbia, Bangladesh, and Brazil. During their production, use, and removal, active pharmaceutical ingredients (APIs), like other synthetic fixings, are delivered into the climate. Drugs (principally restorative items, yet additionally other individual consideration items) may be seen as natural poisons attributed to their usage in both human and veterinary medicine3. Moreover, 559 unique medication trimmings were found in biological territories, such as surface water, groundwater, and soil. Approximately 3000 potent pharmaceutical derivatives have been approved for sale in the EU, with annual per capita therapeutic applications ranging from 50 to 150 grams per capita annually4. Activities involving sewage treatment, surface runoff, soil sifting, and manure application frequently result in the release of pharmaceuticals and metabolites into the marine ecosystem via urine and faeces5. Aquafarming requires veterinary medications, leading to their direct release into the environment. Topical treatments are indirectly related to runoff, manure, and animal feces from fields, which are the primary sources 6. A worldwide survey showed that more than 600 distinctive APIs have been recognized in the climate, sometimes at levels that represent a high danger to the climate7.
Extensive research is being conducted to address harmful pharmaceutical pollutants, which have become a significant concern because of the growing risk they pose to society. Consequently, the number of research publications on this topic has steadily increased, as shown in Figure 1. This study aims to fill the existing gaps in understanding common pharmaceutical pollution and explore the critical role of pharmacists in mitigating these issues. It also examines various regulatory agencies involved in pharmaceutical waste management, offering a comparative analysis of the schedules and guidelines of different countries. Previous studies have often overlooked the comprehensive understanding of the environmental impact of pharmaceutical pollution and the collaborative efforts needed across sectors. This study addresses these gaps by providing a more holistic view of pharmaceutical pollution management and the specific actions required from key regulatory authorities in India.
Inclusion/exclusion criteria
The inclusion and exclusion criteria were designed to ensure the selection of relevant, high-quality, and impactful research. The inclusion criteria focused on peer-reviewed articles, systematic reviews, meta-analyses, and original research that discussed pharmaceutical pollution sources, global health hazards, and preventive strategies, including regulatory measures and the role of pharmacists. The exclusion criteria eliminated non-peer-reviewed or opinion-based articles, studies lacking primary data or methodological rigor, and those limited to unrelated pollutants or isolated local effects without global relevance. This approach ensures a comprehensive review of the global health hazards of pharmaceutical pollution and its mitigation strategies.
.PNG)
Figure 1: Estimated research publications on pharmaceutical pollution and its management
The data were obtained from publications published in different years on pharmaceutical pollution. We focused on recent journals that provide more information. This shows that people are becoming concerned about pharmaceutical pollution and want it to be prevented and investigated.
Materials and Methods
The review was prepared by ensuring that the search criteria for pharmaceutical pollution are critical for ensuring the rigor and relevance of the included literature. A systematic search for relevant literature was performed across multiple reputable databases to gather comprehensive and current information on the management of pharmaceutical pollution. The resources used to obtain appropriate information were PubMed, Google Scholar, Scopus, and Web of Science. A careful assessment was carried out to obtain appropriate information about the global issue of pharmaceutical pollution.
Pharmaceuticals affecting the ecosystem
Determining the impact of synthetic and semi-synthetic pharmaceutical formulations on ecosystems is not easy. Currently, it has become evident that categories of drugs, including antifungal, antibacterial, xeno–estrogens, and anti-parasites, are ecologically toxic and pose environmental threats. Between 1996 and 2007, a significant decline occurred in the vulture population, with millions affected by exposure to anti-inflammatory medicines, resulting in near extinction8,9. Due to the necessity of these treatments for alleviating pain and fever in cattle and the disposal of numerous deceased calves for vulture feeding, certain animals were subjected to elevated doses of diclofenac10,11. A significant percentage of birds died due to severe renal failure and abdominal gout. Vultures from the genus Gyps are significantly susceptible to diclofenac, highlighting the detrimental effects of these pharmaceuticals on avian populations, ultimately jeopardizing three Gyps species in Asia12. Physicians recommend against the concomitant use of ibuprofen and beta-blockers; however, this does not apply to marine fish, which are significantly exposed to various combinations of medications and pollutants, as illustrated in Figure 2. Certain studies have demonstrated the feminizing effects of estrogen, including bisphenol-A, present in contraceptive pills on male fish, which adversely impacts the endocrine system13. River pollution is a major issue affecting the entire ecosystem, including the Yamuna River, which is affected by industrial waste. The worst effect was observed in the Okhla and Nizamuddin bridges, making it the seventh worst-affected river with the highest biochemical oxygen demand. However, certain stretches of the river are free from these pollution impacts, such as the Yamuna River originating from the Tajewala Barrage 14,15. According to reports, the top five polluted rivers in India are as follows:
Ganga River>Yamuna>Brahmaputra>Damodar> Bagmati 16.
.PNG)
Figure 2: Illustration of the transportation of pharmaceuticals used by humans back to humans through a series chain 13.
Common pharmaceuticals detected in water and their environmental and human health effects
Diverse pharmacological sources contribute to the contamination of both surface and groundwaters. Urban wastewater contains a significant concentration of pharmaceuticals derived from human excreta, compounded by the incorrect disposal of expired and unused medications. A significant cause of water contamination is the management of agricultural and livestock waste. Animal waste fed with additives containing pharmaceuticals is often used as a soil amendment and eventually leaches into groundwater 17,18.
Drugs that are found in higher concentrations in wastewater include antibiotics, anti-neoplastic drugs, antiretroviral drugs, endocrine disruptors, analgesics, psychoactive drugs, beta blockers, and NSAIDSs. A detailed description of these pharmaceutical contaminants is provided in
Table 1.
Diverse pharmacological sources contribute to the contamination of both surface and groundwaters. Urban wastewater contains a significant concentration of pharmaceuticals derived from human excreta, compounded by the incorrect disposal of expired and unused medications. A significant cause of water contamination is the management of agricultural and livestock waste. Animal waste fed with additives containing pharmaceuticals is often used as a soil amendment and eventually leaches into groundwater 17,18.
Drugs that are found in higher concentrations in wastewater include antibiotics, anti-neoplastic drugs, antiretroviral drugs, endocrine disruptors, analgesics, psychoactive drugs, beta blockers, and NSAIDSs. A detailed description of these pharmaceutical contaminants is provided in
Table 1.
Table 1: Summary of pharmaceutical contaminants in aquatic environments: concentrations, impacted areas, ecosystem effects, and key observations
| Category | Key Contaminants | Concentration (Range) | Impacted Areas | Effects on Ecosystems and Organisms | Key Observations | References |
| NSAIDs | Diclofenac, Ibuprofen, Naproxen, Indomethacin, Acetaminophen | Diclofenac: Up to 10,221 ng/L (Red Sea, Saudi Arabia) Ibuprofen: 326.1–2,094.4 ng/L (Santos Bay) Acetaminophen: 48.74 ng/L (Antarctica) |
Surface waters: Red Sea, Santos Bay, Antarctic Peninsula |
|
|
19-27 |
| Antimicrobial Drugs | Antibiotics: Ciprofloxacin, Vancomycin, Ampicillin, Tetracyclines, Penicillin |
|
Rivers in China (e.g., Pearl, Hai), Seine River (France), Ebro River (Spain), USA, Italy, South Korea |
|
|
28-44 |
| β-Blockers | Bisoprolol, Propranolol | 3–6,167 ng/L | Surface waters, wastewater treatment plants |
|
|
44-46 |
| Psychoactive Drugs | Opioids, Cannabis, CNS depressants, CNS stimulants, Hallucinogens | Varies | Global water systems |
|
|
44-46 |
| Antiretrovirals | - | Minimal monitoring | Wastewater, drinking water channels |
|
|
47 |
| Anticancer Drugs | Cyclophosphamide, Tamoxifen, Ifosfamide, Methotrexate |
|
Surface water, wastewater, hospital discharges |
|
|
48-53 |
| Endocrinal Drugs | Various medications (e.g., those affecting endogenous hormones) | Not specified | Limited focus on environmental impacts |
|
|
55-57 |
In native ecosystems, there is an impact on the reproductive system, along with changes in vitellogenin levels and hatchability, leading to feminization and posing a significant threat to biodiversity. The category of hazardous waste chemicals, along with their classification codes, is shown in Figure 3.
.PNG)
Figure 3: Classification of Hazardous waste chemicals: This figure explains the categorization of different prominent biohazardous pharmaceuticals, and an attempt has been made to segregate them into different specific classes 78.
Methods to detect traces in water
The prerequisite for progressive approaches to the estimation of a large number of chemicals is the over-reliance on data obtained from various pharmaceuticals and metabolites in environmental media.
Due to the better accuracy and sensitivity of quantification of the contaminants for the polar compounds, there is more preference for “liquid chromatography with triple quadrupole tandem mass spectrometry” 61.
Target analytes usually undergo separation in LC depending on their nature towards the stationary and mobile phases. Once these analytes undergo ionization, they are brought to the “MS/MS detector.” A detailed analysis of the mass-to-charge ratio helps measure the concentrations of pharmaceuticals and their metabolites.
Despite its similarity to state-of-the-art techniques, SPE has some limitations due to variations in the physical and chemical properties of metabolites. Difficulties often arise owing to long runtimes, multiple pre-staging processes, and large solvent volumes, leading to increased capital requirements and reduced sustainability. Such challenges usually come with massive time and cost requirements, leading to major obstacles for some researchers62-63. Therefore, there are absolute restrictions on multi-residue methods for the identification of these metabolites in animal tissues.
Although certain issues have been resolved with the help of technological advancements, such as online solid-phase extraction (SPE) regulations, rapid cleanup of samples and analyte concentrations has been achieved via offline methods.
Moreover, the implementation of ion traps and time-of-flight high-resolution MS for non-target screening augments our understanding of various organic toxins found in environmental samples. Despite advancements in quantity, efficacy, and capacity management for pharmaceutical examination in such trials, the analytical sensitivity and flexibility, as well as the limits of quantitation (LOQs) and limits of detection (LODs), alongside maintenance and operational expenses, remain inferior to offline solid-phase extraction (SPE) and triple quadrupole MS/MS detectors.
Role of pharmacists in controlling pharmaceutical pollution
Pharmacists play a vital role in reducing and controlling drug contamination in the environment. The most ideal approach to alleviate contamination is to prevent drug toxins during the drug manufacturing process. A few organizations have creatively objectified contamination-evading strategies that improve proficiency and benefits while simultaneously limiting ecological effects. “Source diminution” is a strategy by which business plans can be designed to diminish these squanders. For example, measure changes and material replacements may not be as easily executed in the drug business as in other assembling areas. Different drug organizations have recently implemented contamination avoidance programs in their design processes. Several waste reduction initiatives integrate source reduction with recycling and repurposing of garbage to create new and usable products. The tablet coating process involves various methods, including the use of methylene chloride and other chlorinated solvents. Numerous firms have reduced hazardous waste in their air and effluent streams, as well as the cost of purchasing chemicals, by transitioning to fluid-based coating film. Fluid-based cleaning technologies are increasingly employed for equipment cleaning instead of solvent-based techniques65.
A few projects are being carried out where controlled substances and harmful waste prescriptions are acknowledged. When areas gather undesirable prescriptions, the Environmental Protection Agency (EPA) prompts that drugs should be burned at managed places to limit defilement in the environment. As a type of medication organization for the climate, eco-pharmacovigilance (EPV) underscores the source control of drug poisons. Active respondents focused more on the natural issues presented by drug accumulations and became stronger in the EPV intercession, which is mainly carried out by drug ventures and professionals. Accordingly, it is critical to establish standard medication removal conventions and instruct the overall population on the most ideal route for drug removal under the rule of EPV 66.
As indicated by (National Accreditation Board for Hospitals and Healthcare Providers), medications with close/past expiry dates are removed, and no prescriptions with past expiry dates ought to be accessible in the drug store. The emergency clinic ought to characterize what establishes “close to expiry,” for instance, a quarter of a year before the expiry date. To achieve proficiency, the progression of prescriptions should be overseen in all manners to avoid entanglements such as overloading and expiry. The drug store inventory network is the territory where options and bargains are not worthy when inaccessibility emerges.
On the other hand, every pharmacy follows FIFO (First in First Out), which considers the stock that enters the drugstore first and is additionally sold first. This typically occurs when there is a rush due to client requests. The Always, Better, and Control (ABC) investigation is a strategy for grouping things or exercises based on their relative significance. It is otherwise called “isolating the essentials not the many from the many little ones,” because in any collection of elements contributing to a common effect, a small number of participants represents a larger portion of the effects. The VED (Vital, Essential, Desirable) examination depends on the basic qualities and deficiency cost of the item. Because of their criticality, the things could be ordered into three classifications: indispensable, fundamental, and alluring. A Blend of ABC and VED examinations (ABC-VED lattice) can be productively utilized to develop an important command over material supplies 67,68.
Although awareness of the appropriate disposal of drugs is critical, pharmacists and pharmacy students should initiate a prescription reclamation program. Drug specialists should inform patients about how to manage undesirable medicines. Patients should take unused medicines to an office with a physician-recommended drug-reclaim program. Every pharmacy should have a donation site to restore the unused medications with the proper details, such as the bill, and should be used or discarded before expiry to reduce the climate to ruin. Pharmacists as a part of profession should be alert and help the climate for being liberated from its contamination. According to the latest reports, the amount of pharmaceutical waste across the globe ranges from 300,000 tons to 1 million tons, with South Africa having the least and the US having the highest amount of waste (Figure 3) 78.
Health services without Harm (HCWH) of Europe and the European Commission’s Ecological Danger Evaluation of Drug Items (ERAPharm) highlight the issues of false use of drugs and inappropriate disposal of drugs in the climate and challenge the medical care industry to manage them [17]. Similarly, in Australia, the return of unwanted medicines (RUM) project, in Canada, and British Columbia accommodates undesirable and outdated prescriptions to be gathered by local area drug stores. Eleven European :union: countries have drug reclamation frameworks, all of which permit occupants to drop undesirable drugs at drug stores. Estimated data on worldwide waste production are presented in Figure 4. This commitment will create and implement a replicable pilot for gathering undesirable prescriptions at household hazardous waste (HHW) assortments or in other provincial settings70,71. Invert merchants oversee undesirable drugs from drug stores, medical clinics, and facilities. Some of the collected drugs are returned to the manufacturers, while others are bundled and shipped off-site for removal. This administration is painstakingly followed both as a support of the drug business and to meet legitimate prerequisites for the gathered controlled substances and give producer credits to drug stores 71.
The prerequisite for progressive approaches to the estimation of a large number of chemicals is the over-reliance on data obtained from various pharmaceuticals and metabolites in environmental media.
Due to the better accuracy and sensitivity of quantification of the contaminants for the polar compounds, there is more preference for “liquid chromatography with triple quadrupole tandem mass spectrometry” 61.
Target analytes usually undergo separation in LC depending on their nature towards the stationary and mobile phases. Once these analytes undergo ionization, they are brought to the “MS/MS detector.” A detailed analysis of the mass-to-charge ratio helps measure the concentrations of pharmaceuticals and their metabolites.
Despite its similarity to state-of-the-art techniques, SPE has some limitations due to variations in the physical and chemical properties of metabolites. Difficulties often arise owing to long runtimes, multiple pre-staging processes, and large solvent volumes, leading to increased capital requirements and reduced sustainability. Such challenges usually come with massive time and cost requirements, leading to major obstacles for some researchers62-63. Therefore, there are absolute restrictions on multi-residue methods for the identification of these metabolites in animal tissues.
Although certain issues have been resolved with the help of technological advancements, such as online solid-phase extraction (SPE) regulations, rapid cleanup of samples and analyte concentrations has been achieved via offline methods.
Moreover, the implementation of ion traps and time-of-flight high-resolution MS for non-target screening augments our understanding of various organic toxins found in environmental samples. Despite advancements in quantity, efficacy, and capacity management for pharmaceutical examination in such trials, the analytical sensitivity and flexibility, as well as the limits of quantitation (LOQs) and limits of detection (LODs), alongside maintenance and operational expenses, remain inferior to offline solid-phase extraction (SPE) and triple quadrupole MS/MS detectors.
Role of pharmacists in controlling pharmaceutical pollution
Pharmacists play a vital role in reducing and controlling drug contamination in the environment. The most ideal approach to alleviate contamination is to prevent drug toxins during the drug manufacturing process. A few organizations have creatively objectified contamination-evading strategies that improve proficiency and benefits while simultaneously limiting ecological effects. “Source diminution” is a strategy by which business plans can be designed to diminish these squanders. For example, measure changes and material replacements may not be as easily executed in the drug business as in other assembling areas. Different drug organizations have recently implemented contamination avoidance programs in their design processes. Several waste reduction initiatives integrate source reduction with recycling and repurposing of garbage to create new and usable products. The tablet coating process involves various methods, including the use of methylene chloride and other chlorinated solvents. Numerous firms have reduced hazardous waste in their air and effluent streams, as well as the cost of purchasing chemicals, by transitioning to fluid-based coating film. Fluid-based cleaning technologies are increasingly employed for equipment cleaning instead of solvent-based techniques65.
A few projects are being carried out where controlled substances and harmful waste prescriptions are acknowledged. When areas gather undesirable prescriptions, the Environmental Protection Agency (EPA) prompts that drugs should be burned at managed places to limit defilement in the environment. As a type of medication organization for the climate, eco-pharmacovigilance (EPV) underscores the source control of drug poisons. Active respondents focused more on the natural issues presented by drug accumulations and became stronger in the EPV intercession, which is mainly carried out by drug ventures and professionals. Accordingly, it is critical to establish standard medication removal conventions and instruct the overall population on the most ideal route for drug removal under the rule of EPV 66.
As indicated by (National Accreditation Board for Hospitals and Healthcare Providers), medications with close/past expiry dates are removed, and no prescriptions with past expiry dates ought to be accessible in the drug store. The emergency clinic ought to characterize what establishes “close to expiry,” for instance, a quarter of a year before the expiry date. To achieve proficiency, the progression of prescriptions should be overseen in all manners to avoid entanglements such as overloading and expiry. The drug store inventory network is the territory where options and bargains are not worthy when inaccessibility emerges.
On the other hand, every pharmacy follows FIFO (First in First Out), which considers the stock that enters the drugstore first and is additionally sold first. This typically occurs when there is a rush due to client requests. The Always, Better, and Control (ABC) investigation is a strategy for grouping things or exercises based on their relative significance. It is otherwise called “isolating the essentials not the many from the many little ones,” because in any collection of elements contributing to a common effect, a small number of participants represents a larger portion of the effects. The VED (Vital, Essential, Desirable) examination depends on the basic qualities and deficiency cost of the item. Because of their criticality, the things could be ordered into three classifications: indispensable, fundamental, and alluring. A Blend of ABC and VED examinations (ABC-VED lattice) can be productively utilized to develop an important command over material supplies 67,68.
Although awareness of the appropriate disposal of drugs is critical, pharmacists and pharmacy students should initiate a prescription reclamation program. Drug specialists should inform patients about how to manage undesirable medicines. Patients should take unused medicines to an office with a physician-recommended drug-reclaim program. Every pharmacy should have a donation site to restore the unused medications with the proper details, such as the bill, and should be used or discarded before expiry to reduce the climate to ruin. Pharmacists as a part of profession should be alert and help the climate for being liberated from its contamination. According to the latest reports, the amount of pharmaceutical waste across the globe ranges from 300,000 tons to 1 million tons, with South Africa having the least and the US having the highest amount of waste (Figure 3) 78.
Health services without Harm (HCWH) of Europe and the European Commission’s Ecological Danger Evaluation of Drug Items (ERAPharm) highlight the issues of false use of drugs and inappropriate disposal of drugs in the climate and challenge the medical care industry to manage them [17]. Similarly, in Australia, the return of unwanted medicines (RUM) project, in Canada, and British Columbia accommodates undesirable and outdated prescriptions to be gathered by local area drug stores. Eleven European :union: countries have drug reclamation frameworks, all of which permit occupants to drop undesirable drugs at drug stores. Estimated data on worldwide waste production are presented in Figure 4. This commitment will create and implement a replicable pilot for gathering undesirable prescriptions at household hazardous waste (HHW) assortments or in other provincial settings70,71. Invert merchants oversee undesirable drugs from drug stores, medical clinics, and facilities. Some of the collected drugs are returned to the manufacturers, while others are bundled and shipped off-site for removal. This administration is painstakingly followed both as a support of the drug business and to meet legitimate prerequisites for the gathered controlled substances and give producer credits to drug stores 71.
.PNG)
Figure 4: Worldwide estimated waste production data: This demonstrates that the southern region of Africa is creating pharmaceutical pollution, which might be due to less concern about its harmful effects on the environment. It also shows that along with India, China, North, and South America are also playing an important role in adding pollutants to the environment 70,71.
Pharmaceutical polluted water management
In recent years, many studies have shown the process of managing various chemicals from pharmaceuticals and other sources that are associated with adverse effects on the endocrine system. Most of them are concerned with wastewater treatment, which involves various processes, including chemical-based and fermentation-based processes. Single-stage treatment usually comes with the disadvantage of being insufficiently treated, leading to the evolution of hybrid technology, which focuses on the initial treatment to remove non-biodegradable recalcitrant or refractory compounds to increase the remaining bioavailability, so that further biological treatment can be performed. There are two types of biodegradable waste treatment: aerobic and anaerobic bioreactors, which have been helpful in multiple applications, such as the generation of biogas used in the agriculture industry. The waste management techniques are listed in Table 2. Earlier technologies mainly focused on removal, but now, with the changing notion, more focus has been shifted to recovery technology for the recovery of vital reagents, substances, or by-products that have the potential to be reused 72,73.
In recent years, many studies have shown the process of managing various chemicals from pharmaceuticals and other sources that are associated with adverse effects on the endocrine system. Most of them are concerned with wastewater treatment, which involves various processes, including chemical-based and fermentation-based processes. Single-stage treatment usually comes with the disadvantage of being insufficiently treated, leading to the evolution of hybrid technology, which focuses on the initial treatment to remove non-biodegradable recalcitrant or refractory compounds to increase the remaining bioavailability, so that further biological treatment can be performed. There are two types of biodegradable waste treatment: aerobic and anaerobic bioreactors, which have been helpful in multiple applications, such as the generation of biogas used in the agriculture industry. The waste management techniques are listed in Table 2. Earlier technologies mainly focused on removal, but now, with the changing notion, more focus has been shifted to recovery technology for the recovery of vital reagents, substances, or by-products that have the potential to be reused 72,73.
Table 2: Detailed information on managing different categories81.
| Category of waste | Waste Specification | Container Specification | Treatment Characterization |
| BMP toxic and hazardous toxic | D, U and toxic P wastes, non-listed chemotherapy bulk drugs, and toxic drugs PPE with perceptible pollution. | Black | Ignition at Resource Conservation and Recovery Act (RCRA) hazardous waste premises. |
| Hazardous combustible | D001 wastes | Black | Incineration at RCRA hazardous waste premises. |
| Hazardous and infectious | If P-listed hazardous waste was disposed of in a container properly or improperly, hazardous toxic wastes and BMP toxic wastes in combination with RMW the entire contents of sharps containers (NOTE: Recent expansion of the epinephrine syringe exemption should reduce this waste to a minimum if accepted by states.) | Black hazardous waste container in needle box configuration with RMW label applied | Combustion at a site approved to manage RCRA hazardous waste and RMW. |
| Trace chemotherapy | "RCRA" empty vials of chemotherapy chemicals, needles and syringes, IVs, and personal protective equipment (PPE) used to prepare or administer chemotherapy that are unremarkably contaminated | Yellow | Incineration at RMW premises |
| Drain disposal | Vitamins, electrolytes, sodium chloride, dextrose, and controlled substances. | Sewer NOTE: Prohibition on disposal of controlled and hazardous substances in drainage | Local POTW (permission required) |
| MP Uncontrolled | All other drugs | Cream with purple top or white with blue top | Incineration at RMW or municipal solid waste (MSW) facility |
Regulatory bodies for managing pharmaceutical wastes
The US Environmental Protection Act regulates pharmaceutical household waste. The Resource Conservation and Recovery Act (RCRA) covers waste from pharmaceuticals and other hazardous products. It only governs pharmaceutical waste, which is an unsorted collection of unwanted medications, some of which may be utilized in the future or processed and reused. The reverse distribution industry is built on this foundation of sustainability. The reverse distribution of pharmaceutical waste has also been approved by the US EPA. According to the U.S. EPA permits and waste management systems must be addressed by the industry75.
US Drug Enforcement Administration (DEA): The DEA of the United States regulates some pharmaceuticals, which are referred to as “controlled substances.” These are crucial for certain medicines that can be abused, such as opioids and tranquilizers such as codeine, Valium, Ritalin, anabolic steroids, and Lomotil. Five schedules were used by the DEA to list the prohibited drugs. Medications listed in Schedules II through V are prescribed to patients, while medications listed in Schedule I have no therapeutic benefit.
Health Insurance Portability and Accountability Act (HIPAA): A standard for safeguarding the privacy of personal health information is established by the HIPAA, which is managed by the U.S. Department of Health and Human Services (DHHS). Prescription labels and other personally identifiable medical information must be secured according to these standards. In theory, HIPAA only protects businesses associated with medical care providers, including pharmacies and insurance companies. Pharmacies may collect waste from business associates. According to HIPAA regulations, waste management contractors are recognized as business associates, and to safeguard confidential patient data, the pharmacy and waste management contractor must sign a written agreement76. The schedules and guidelines for managing pharmaceuticals are presented in Table 3.
The US Environmental Protection Act regulates pharmaceutical household waste. The Resource Conservation and Recovery Act (RCRA) covers waste from pharmaceuticals and other hazardous products. It only governs pharmaceutical waste, which is an unsorted collection of unwanted medications, some of which may be utilized in the future or processed and reused. The reverse distribution industry is built on this foundation of sustainability. The reverse distribution of pharmaceutical waste has also been approved by the US EPA. According to the U.S. EPA permits and waste management systems must be addressed by the industry75.
US Drug Enforcement Administration (DEA): The DEA of the United States regulates some pharmaceuticals, which are referred to as “controlled substances.” These are crucial for certain medicines that can be abused, such as opioids and tranquilizers such as codeine, Valium, Ritalin, anabolic steroids, and Lomotil. Five schedules were used by the DEA to list the prohibited drugs. Medications listed in Schedules II through V are prescribed to patients, while medications listed in Schedule I have no therapeutic benefit.
Health Insurance Portability and Accountability Act (HIPAA): A standard for safeguarding the privacy of personal health information is established by the HIPAA, which is managed by the U.S. Department of Health and Human Services (DHHS). Prescription labels and other personally identifiable medical information must be secured according to these standards. In theory, HIPAA only protects businesses associated with medical care providers, including pharmacies and insurance companies. Pharmacies may collect waste from business associates. According to HIPAA regulations, waste management contractors are recognized as business associates, and to safeguard confidential patient data, the pharmacy and waste management contractor must sign a written agreement76. The schedules and guidelines for managing pharmaceuticals are presented in Table 3.
Table 3: Schedule and guidelines79
| Schedule code | Regulatory guidelines |
| Schedule 1 | Biomedical waste handling and disposal. |
| Schedule 2 | Waste is separated and placed in various bags or containers. |
| Schedule 3 | Each container has a label. |
According to regulatory guidelines, chemical substances are categorized into different categories, each with a specific code. Among the various categories, some important ones are shown in Figure 3. The Code (40 CFR Part 261.33I) represents P-Listed Wastes, which are products categorized as acutely hazardous under the RCRA because of their oral lethal dose of 50 mg/kg (LD50) or less. A total of eight compounds were P-listed. Nitroglycerin and adrenaline are the two most commonly used P-listed compounds. Each of these has exceptions offered by the USEPA and the EPA of numerous states. Federal law prohibits the use of epinephrine salts as of October 15, 2007, and weak medical nitroglycerin as of August 14, 200163,64.
The code 40 CFR Part 261.33(f) represents U-Listed Wastes, containing 21 drugs listed in the U-list because of their toxicity. Under the following specified conditions, a drug must be classified as hazardous waste:
The code 40 CFR Part 261.33(f) represents U-Listed Wastes, containing 21 drugs listed in the U-list because of their toxicity. Under the following specified conditions, a drug must be classified as hazardous waste:
- Relinquished medication waste comprises a single dynamic component from the U list: it was not used for its authorized purpose. There are no concentration limits or dilution exclusions for P-listed wastes80.
ii. Blank receptacle of U-listed waste (40 CFR Part 261.7(b)(1)). Two conditions must be met for a container to be designated as “RCRA empty” after holding U-listed waste:
(1) Normal methods, such as syringes, were used to extract all contents.
(2) A maximum of 3% by mass of remnants.
A container is classified as hazardous waste if neither of these conditions is satisfied. The code 40 CFR Part 261.24 represents multiple D codes with 40 chemicals in the list comprised in RCRA as a concern in a solid waste landfill atmosphere above the specified percentage. Waste exceeding these amounts must be treated as unsafe. The Toxicity Characteristic Leaching Procedure (TCLP) is a test that measures the percolation of certain substances and heavy metallic elements in a landfill setting. If the concentration determined by the TCLP exceeds the specified limitations, the waste must be treated as hazardous81.
Guidelines and Practices for Controlling Pharmaceutical Pollution 78,79: It is essential to formulate new pharmaceutical waste management policies and procedures following the completion of a pilot project and prior to the implementation of the program across the entire facility. The management of nursing, safety, environmental services, and pharmacies plays a crucial role in formulating these new recommendations. If required, new regulations and protocols should be established, or all elements of pharmaceutical waste management and minimization programs should be integrated into existing frameworks, as outlined in Table 4 and Table 5. Additionally, consolidating these efforts into a singular, comprehensive rulebook for pharmaceutical waste management and reduction policies and processes would be advantageous. The production, administration, and disposal of chemotherapy, as well as general hazardous waste management, exemplify further pertinent regulations and protocols that may be referenced in this comprehensive operating manual73.
Policies and procedures for pharmaceutical waste management should be created to specify the organizational strategy for82-84:
(1) Normal methods, such as syringes, were used to extract all contents.
(2) A maximum of 3% by mass of remnants.
A container is classified as hazardous waste if neither of these conditions is satisfied. The code 40 CFR Part 261.24 represents multiple D codes with 40 chemicals in the list comprised in RCRA as a concern in a solid waste landfill atmosphere above the specified percentage. Waste exceeding these amounts must be treated as unsafe. The Toxicity Characteristic Leaching Procedure (TCLP) is a test that measures the percolation of certain substances and heavy metallic elements in a landfill setting. If the concentration determined by the TCLP exceeds the specified limitations, the waste must be treated as hazardous81.
Guidelines and Practices for Controlling Pharmaceutical Pollution 78,79: It is essential to formulate new pharmaceutical waste management policies and procedures following the completion of a pilot project and prior to the implementation of the program across the entire facility. The management of nursing, safety, environmental services, and pharmacies plays a crucial role in formulating these new recommendations. If required, new regulations and protocols should be established, or all elements of pharmaceutical waste management and minimization programs should be integrated into existing frameworks, as outlined in Table 4 and Table 5. Additionally, consolidating these efforts into a singular, comprehensive rulebook for pharmaceutical waste management and reduction policies and processes would be advantageous. The production, administration, and disposal of chemotherapy, as well as general hazardous waste management, exemplify further pertinent regulations and protocols that may be referenced in this comprehensive operating manual73.
Policies and procedures for pharmaceutical waste management should be created to specify the organizational strategy for82-84:
- Identifying drugs that must be managed as hazardous waste
- Determining which non-regulated drugs will be managed as hazardous waste
- Maintaining a system to add new drugs
- Labeling drugs to facilitate the segregation of hazardous waste
- Segregating waste streams
- Training staff (e.g., which staff, what information, and how often)
- Managing spills;
- Contacting emergency coordinators.
- Establishing and managing satellite storage and accumulation zones
- Generating and maintaining manifests for potentially hazardous wastes
- Determining the hazardous waste generation status
- What criteria are used for hazardous waste selection?
- Scheduling regular program reviews
- Updating management on relevant matters; and
- Leveraging pharmaceutical waste management as a foundation for a comprehensive Environmental Management System (EMS) throughout the facility.
Table 4: Schedules and guidelines of different countries on pharmaceutical waste management 76-79.
| S.No. | Country | Guidelines/Regulations | Schedules | Key Points | Role | Reference |
| 1 | United States | RCRA | Schedule I-V for controlled substances | Detailed protocols for disposal and handling of hazardous pharmaceutical waste | Oversees the management of hazardous and pharmaceutical waste under RCRA | EPA RCRA Guidelines |
| 2 | Canada | Canadian Environmental Protection Act (CEPA) | Schedule I for controlled substances | Regulations for pharmaceutical waste management, including disposal and treatment | Regulates pharmaceutical waste under CEPA | Canada.ca Environmental Regulations |
| 3 | United Kingdom | Hazardous Waste Regulations (HWR) | List of Waste (LoW) Codes | Guidelines for classification and disposal of hazardous pharmaceutical waste | Enforces HWR and provides guidelines for pharmaceutical waste | UK Environment Agency |
| 4 | European :union: | Waste Framework Directive (WFD) and EU Regulations | European Waste Catalogue (EWC) Codes | Comprehensive framework for handling and disposal of pharmaceutical waste | Develops and implements directives on waste management including pharmaceuticals | European Commission Waste |
| 5 | Australia | National environment protection (used packaging materials) act | Schedule for pharmaceutical waste | Guidelines for managing pharmaceutical waste, including segregation and disposal | Manages pharmaceutical waste under national environmental protection laws | Australian Government Department of Agriculture, Water and the Environment |
| 6 | India | Biomedical Waste Management Rules | Schedule I for Bio-Medical Waste | Guidelines for the collection, treatment, and disposal of pharmaceutical waste | Regulates pharmaceutical waste under Biomedical Waste Management Rules | Ministry of Environment, Forest and Climate Change, India |
| 7 | Japan | Waste Management and Public Cleansing Law | General and Special Waste Categories | Rules for managing pharmaceutical waste and promoting recycling | Oversees waste management including pharmaceutical waste under the Waste Management and Public Cleansing Law | Japan Ministry of the Environment |
It can be inferred that the management of pharmaceutical contamination differs markedly among nations and is influenced by distinct legal frameworks, implementation methodologies, and environmental objectives. The RCRA in the United States classifies pharmaceutical waste into five categories and establishes comprehensive standards for the management of hazardous waste under the jurisdiction of the EPA. Canada enforces the CEPA, which prioritizes stringent restrictions for the disposal and treatment of pharmaceutical waste, albeit with a somewhat limited scope than the U.S. framework. The European :union: implements a holistic strategy via the WFD and EWC rules, guaranteeing consistent waste management standards among the member states. The United Kingdom enhances this with its HWR, which includes comprehensive classification and disposal directives. Australia implements pharmaceutical waste management through the National Environment Protection Act, highlighting the importance of segregation and appropriate disposal following environmental protection legislation. Conversely, nations such as India and Japan depend on comprehensive regulatory frameworks, including the Biomedical Waste Management Rules and Waste Management and Public Cleansing Law, respectively. These guidelines focus on general waste categories, including pharmaceutical waste as a subset, thereby creating deficiencies in specialized treatment procedures. These disparities emphasize the necessity for the global standardization of pharmaceutical pollution control to guarantee uniform protection of ecosystems and public health.
Table 5: Tabular representation of various regulatory bodies across the world 76-79
| Country | Regulation Name | Regulating body |
| USA | Resource Conservation and Recovery Act (RCRA) | Environmental Protection Agency (EPA) |
| Canada | Canadian Environmental Act (CEPA) | Environment and Climate Change Canada |
| India | Biomedical Waste Management Rules | Ministry of Environment, Forest and Climate Change (MoEFCC) |
| Japan | Waste Management and Public Cleansing Law | Ministry of the Environment |
| Australia | National Environment Protection (Used Packaging Materials Act) | Department of Agriculture, Water and the Environment |
| European :union: | Waste Framework Directive and EU Regulation | European Commission |
| United Kingdom | Hazardous Waste Regulation (HWR) | Environment Agency |
Emerging pharmaceutical manufacturing countries (EPMCs) have numerous potential advantages, including expanding markets, streamlined clinical trials, and reduced production costs. The migration and expansion of the pharmaceutical sector in EPMCs have positioned them as the leading consumers of pharmaceuticals globally. The issue of contamination from pharmaceuticals in EPMCs is intricate due to elevated concentrations of pharmaceutical chemicals and a lack of sufficient technical resources and expertise for surveillance and care, in contrast to wealthy nations85. In developed nations, pharmaceutical products are primarily released as point sources due to extensive wastewater connectivity. Conversely, diffuse pollution poses a greater issue in numerous nations with low or middle incomes owing to spills from sewers and wastewater treatment plants and the dumping of untreated sewage or filtrate86. Many regions with limited or non-existent research are growing nations; thus, medicinal product demand and associated pollutant profiles may significantly differ from those of advanced nations. In nations with developing economies, managing water resources may not have advanced to the point where pollution detection and contaminant management are adequately prioritized87. Developed nations generally possess sophisticated wastewater treatment facilities that utilize methods such as activated carbon filters and complex oxidation procedures, which can efficiently diminish pharmaceutical contaminants in water. They possess comprehensive pharmaceutical disposal and collection systems bolstered by public outreach initiatives advocating appropriate disposal techniques. Conversely, pharmerging nations frequently lack adequate infrastructure, with numerous areas depending on rudimentary wastewater treatment that is incapable of eliminating pharmaceutical pollutants85,86,88.
Results
The overall study showed alarming hazards due to pharmaceutical pollution. Currently, there is a dearth of research on how drug contamination affects human health. According to a 2012 WHO report, drug concentrations in tap water should not have any negative health impact. A follow-up study confirmed that these results were obtained in China. However, the most susceptible patient groups (e.g., those with allergies) may experience issues as a result of environmental medication exposure. Despite the lack of evidence for any short-term adverse effects on human health, many issues remain, particularly concerning long-term (chronic) exposure to various pollutants. The main foods and beverages that contain these medications include vegetables, meat, fish, dairy products, and tubers. This review provides a complete overview of countries with high rates of pharmaceutical production, which are much more susceptible to environmental pollution. They should follow the guidelines along with preventive measures.
Discussion
The current assessment indicates that the drugstore industry and its global employees play a significant role in preventing pharmaceutical contamination of the atmosphere. However, there are effective monitoring programs and comprehensive, accurate studies on the occurrence of drugs in drinking water. One of the major challenges in assessing the possible risks associated with tracking drug groups in drinking water is the lack of information. Although ebb and flow hazard assessments show that low concentrations of drugs in drinking water are unlikely to pose any risks to human health, there are gaps in knowledge regarding the risks associated with long-term, low-level drug exposure and the potential combined effects of compound combinations, including drugs. Encouragement to invest in and work effectively on wastewater innovation is essential to ensure safe release levels from drug production. Financial incentives, such as legal commitments that may result in fines or cancellation of operating licenses, are the most compelling. Their focus on customers is essential for achieving the goal of a pharmacy-free environment. Both private and hospital pharmacies need to display prominent banners that make it simple for customers to return unused medications, regardless of the store where they were acquired, as long as they follow the correct guidelines. Pharmacies should continue to reduce medication expiration dates using VED-ABC and FIFO-FEFO analyses. Pharmacists must advise patients and accompanying caregivers on how to properly dispose of medications. As a result of increased awareness, several programs have been launched to better understand the environmental effects of pharmaceutical production and how to reduce them. Adopting a cost-effective system is necessary to provide better medical care. A new system must be implemented to guarantee appropriate waste management and reduce waste generation by raising awareness and educating all parties involved. Pharmaceutical waste discharge into water and pharmaceutical-treated contaminated water must be given special attention to ensure that the water is fit for human consumption. Healthcare facilities, patients, and the general public should be able to return expired and undesired medications for safe disposal in waste-to-energy or incinerator facilities, provided that the necessary legal framework and logistical resources are in place. Protesters hope that local organizations directly impacted by the pollution caused by the pharmaceutical business will exert pressure on the industry to keep its unfulfilled pledges to lessen the negative environmental effects of medication production. Through appropriate return policies and donation management, as well as appropriate sales and a decrease in medication expiration, the production of pharmaceuticals will also be reduced.
Conclusion
The current review showed that the pharmacy industry and pharmacists worldwide are working to keep climate contamination liberated from pharmaceutical items. This area of research is still being consideration [23-25]. FIFO – FEFO and VED – ABC analyses are promising methods for reducing pharmacy waste. There is a need to implement cost-effective systems to enhance medical treatment facilities while ensuring proper waste management through the adoption of new systems and reducing waste generation through awareness and education initiatives involving all stakeholders. Special concern on the waste disposal of pharmaceuticals into the water and the treatment of polluted water by pharmaceuticals is to be taken to make the water safe for use. Additional exploration of the impact of exposure to drug combinations would be beneficial, particularly when paired with monitoring initiatives. Such research could contribute to creating awareness among individuals involved in pharmacy and medicine, ultimately aiding in enhancing environmental quality by reducing pharmaceutical pollution. Furthermore, it could facilitate the development of data for human health peril evaluation and ecotoxicological risk analysis associated with pharmaceutical pollutants. Therefore, this study integrates environmental science, healthcare, and politics, highlighting an interdisciplinary approach to managing pharmaceutical pollution, in contrast to studies that concentrate solely on one discipline. This study emphasizes the significance of pharmacists in addressing pharmaceutical pollution, a sometimes-neglected aspect in comparable research. A comparative analysis of regulatory schedules and recommendations across nations offers a global perspective, emphasizing disparities and exemplary practices that other studies may overlook. Despite the aforementioned aspects, this study had specific limitations compared to other studies. This study may focus on ecosystem impacts without extensively exploring specific subtopics such as bioaccumulation. A comparison of regulatory schedules among countries may not fully account for the impact of cultural, economic, or infrastructural disparities on implementation and efficacy. The study may restrict its discourse on preventive measures to conventional techniques, neglecting novel approaches such as green pharmaceutical practices or AI-driven solutions. Finally, when regulatory agencies are examined, the analysis of enforcement issues or historical trends may be less thorough than in other studies, which often offer more extensive policy evaluations and contextual insights.
Acknowledgements
The researchers acknowledge Galgotias University, Greater Noida, and Jimma University College of Agriculture and Veterinary Medicine for providing the facilities to conduct this study. This study is part of the PhD thesis of the first author. We also acknowledge the Ethiopian Meteorological Institute (EMI) and National Aeronautics and Space Administration (NASA) for providing climate data at no cost.
Conflict of Interest
There are no conflicts of interest to declare. The entire review was a team effort.
Funding
Self-funded.
Ethical Considerations
Not applicable, as this is a review article.
Code of Ethics
Not applicable, as this is a review article.
Author contributions
Sanjita Das: Conceptualization, Editing.
Renu Tushir: Conceptualization, methodology, resources, data curation writing—original draft preparation, visualization
Ajesh Chahuan: Pictorial framing, investigation, resources, writing—review and editing,
Saurabh Gurjar: writing and editing,
Lakshay Bhanwala: Collecting information, conceptualization, editing
Sneha Patnaik: Editing grammar
Anushka Jain: Editing and information collection.
All authors have read and agreed to the published version of the manuscript.
Abbreviations
PPCPs: Pharmaceutical and personal care products
CEPA- Canadian Environmental Protection Act
EU- European :union:
APIs: Active Pharmaceutical Ingredients
NSAIDSs: Non-steroidal Anti-inflammatory Drugs and Analgesics
LOQs- limits of Quantitation
LODs- Limits of detection
SPE- solid-phase extraction
MS- Mass Spectroscopy
LC- Liquid Chromatography
CFR- Code of Federal Regulation
EPA- Environment Protection Agency
EPV- Ecopharmacovigilance
ABC- Always, Better, Control
VED- Vital, Essential, Desirable
RUM-Return unwanted medicine
HHW- Household hazardous waste
NABH- National Accreditation Board for Hospitals and Healthcare Providers
FIFO- First in First Out
HCHW- Health Control without Harm
DHHS- Department of Health and Human Services
DEA- Drug Enforcement Administration
HIPPA- Health Insurance Portability and Accountability Act
TCLP- Toxicity Characteristic Leaching Procedure
EMS- Environmental Management System
WFD- Waste Framework Directive
RCRA- Resource Conservation and Recovery Act
HWR- Hazardous Waste Regulation
Disclosure statement
The authors declare no potential conflicts of interest.
Data availability statement
No other data, apart from those already included in the manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
Results
The overall study showed alarming hazards due to pharmaceutical pollution. Currently, there is a dearth of research on how drug contamination affects human health. According to a 2012 WHO report, drug concentrations in tap water should not have any negative health impact. A follow-up study confirmed that these results were obtained in China. However, the most susceptible patient groups (e.g., those with allergies) may experience issues as a result of environmental medication exposure. Despite the lack of evidence for any short-term adverse effects on human health, many issues remain, particularly concerning long-term (chronic) exposure to various pollutants. The main foods and beverages that contain these medications include vegetables, meat, fish, dairy products, and tubers. This review provides a complete overview of countries with high rates of pharmaceutical production, which are much more susceptible to environmental pollution. They should follow the guidelines along with preventive measures.
Discussion
The current assessment indicates that the drugstore industry and its global employees play a significant role in preventing pharmaceutical contamination of the atmosphere. However, there are effective monitoring programs and comprehensive, accurate studies on the occurrence of drugs in drinking water. One of the major challenges in assessing the possible risks associated with tracking drug groups in drinking water is the lack of information. Although ebb and flow hazard assessments show that low concentrations of drugs in drinking water are unlikely to pose any risks to human health, there are gaps in knowledge regarding the risks associated with long-term, low-level drug exposure and the potential combined effects of compound combinations, including drugs. Encouragement to invest in and work effectively on wastewater innovation is essential to ensure safe release levels from drug production. Financial incentives, such as legal commitments that may result in fines or cancellation of operating licenses, are the most compelling. Their focus on customers is essential for achieving the goal of a pharmacy-free environment. Both private and hospital pharmacies need to display prominent banners that make it simple for customers to return unused medications, regardless of the store where they were acquired, as long as they follow the correct guidelines. Pharmacies should continue to reduce medication expiration dates using VED-ABC and FIFO-FEFO analyses. Pharmacists must advise patients and accompanying caregivers on how to properly dispose of medications. As a result of increased awareness, several programs have been launched to better understand the environmental effects of pharmaceutical production and how to reduce them. Adopting a cost-effective system is necessary to provide better medical care. A new system must be implemented to guarantee appropriate waste management and reduce waste generation by raising awareness and educating all parties involved. Pharmaceutical waste discharge into water and pharmaceutical-treated contaminated water must be given special attention to ensure that the water is fit for human consumption. Healthcare facilities, patients, and the general public should be able to return expired and undesired medications for safe disposal in waste-to-energy or incinerator facilities, provided that the necessary legal framework and logistical resources are in place. Protesters hope that local organizations directly impacted by the pollution caused by the pharmaceutical business will exert pressure on the industry to keep its unfulfilled pledges to lessen the negative environmental effects of medication production. Through appropriate return policies and donation management, as well as appropriate sales and a decrease in medication expiration, the production of pharmaceuticals will also be reduced.
Conclusion
The current review showed that the pharmacy industry and pharmacists worldwide are working to keep climate contamination liberated from pharmaceutical items. This area of research is still being consideration [23-25]. FIFO – FEFO and VED – ABC analyses are promising methods for reducing pharmacy waste. There is a need to implement cost-effective systems to enhance medical treatment facilities while ensuring proper waste management through the adoption of new systems and reducing waste generation through awareness and education initiatives involving all stakeholders. Special concern on the waste disposal of pharmaceuticals into the water and the treatment of polluted water by pharmaceuticals is to be taken to make the water safe for use. Additional exploration of the impact of exposure to drug combinations would be beneficial, particularly when paired with monitoring initiatives. Such research could contribute to creating awareness among individuals involved in pharmacy and medicine, ultimately aiding in enhancing environmental quality by reducing pharmaceutical pollution. Furthermore, it could facilitate the development of data for human health peril evaluation and ecotoxicological risk analysis associated with pharmaceutical pollutants. Therefore, this study integrates environmental science, healthcare, and politics, highlighting an interdisciplinary approach to managing pharmaceutical pollution, in contrast to studies that concentrate solely on one discipline. This study emphasizes the significance of pharmacists in addressing pharmaceutical pollution, a sometimes-neglected aspect in comparable research. A comparative analysis of regulatory schedules and recommendations across nations offers a global perspective, emphasizing disparities and exemplary practices that other studies may overlook. Despite the aforementioned aspects, this study had specific limitations compared to other studies. This study may focus on ecosystem impacts without extensively exploring specific subtopics such as bioaccumulation. A comparison of regulatory schedules among countries may not fully account for the impact of cultural, economic, or infrastructural disparities on implementation and efficacy. The study may restrict its discourse on preventive measures to conventional techniques, neglecting novel approaches such as green pharmaceutical practices or AI-driven solutions. Finally, when regulatory agencies are examined, the analysis of enforcement issues or historical trends may be less thorough than in other studies, which often offer more extensive policy evaluations and contextual insights.
Acknowledgements
The researchers acknowledge Galgotias University, Greater Noida, and Jimma University College of Agriculture and Veterinary Medicine for providing the facilities to conduct this study. This study is part of the PhD thesis of the first author. We also acknowledge the Ethiopian Meteorological Institute (EMI) and National Aeronautics and Space Administration (NASA) for providing climate data at no cost.
Conflict of Interest
There are no conflicts of interest to declare. The entire review was a team effort.
Funding
Self-funded.
Ethical Considerations
Not applicable, as this is a review article.
Code of Ethics
Not applicable, as this is a review article.
Author contributions
Sanjita Das: Conceptualization, Editing.
Renu Tushir: Conceptualization, methodology, resources, data curation writing—original draft preparation, visualization
Ajesh Chahuan: Pictorial framing, investigation, resources, writing—review and editing,
Saurabh Gurjar: writing and editing,
Lakshay Bhanwala: Collecting information, conceptualization, editing
Sneha Patnaik: Editing grammar
Anushka Jain: Editing and information collection.
All authors have read and agreed to the published version of the manuscript.
Abbreviations
PPCPs: Pharmaceutical and personal care products
CEPA- Canadian Environmental Protection Act
EU- European :union:
APIs: Active Pharmaceutical Ingredients
NSAIDSs: Non-steroidal Anti-inflammatory Drugs and Analgesics
LOQs- limits of Quantitation
LODs- Limits of detection
SPE- solid-phase extraction
MS- Mass Spectroscopy
LC- Liquid Chromatography
CFR- Code of Federal Regulation
EPA- Environment Protection Agency
EPV- Ecopharmacovigilance
ABC- Always, Better, Control
VED- Vital, Essential, Desirable
RUM-Return unwanted medicine
HHW- Household hazardous waste
NABH- National Accreditation Board for Hospitals and Healthcare Providers
FIFO- First in First Out
HCHW- Health Control without Harm
DHHS- Department of Health and Human Services
DEA- Drug Enforcement Administration
HIPPA- Health Insurance Portability and Accountability Act
TCLP- Toxicity Characteristic Leaching Procedure
EMS- Environmental Management System
WFD- Waste Framework Directive
RCRA- Resource Conservation and Recovery Act
HWR- Hazardous Waste Regulation
Disclosure statement
The authors declare no potential conflicts of interest.
Data availability statement
No other data, apart from those already included in the manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
- Zhang Y, Duan L, Wang B, et al. Efficient multiresidue determination method for 168 pharmaceuticals and metabolites: optimization and application to raw wastewater, wastewater effluent, and surface water in Beijing, China. Environmental Pollution. 2020;261:114113.
- Čelić MF, Alda ML, Perez S, et al. Environmental analysis: emerging pollutants. InLiquid Chromatography. Elsevier; 2023. p. 549-78.
- Boxall AB, Rudd MA, Brooks BW, et al. Pharmaceuticals and personal care products in the environment: what are the big questions?. Environ Health Perspect. 2012;120(9):1221-9.
- Causanilles A, Baz-Lomba JA, Burgard DA, et al. Improving wastewater-based epidemiology to estimate cannabis use: focus on the initial aspects of the analytical procedure. Anal Chim Acta. 2017;988:27-33.
- Iloms E, Ololade OO, Ogola HJO, et al. Investigating industrial effluent impact on municipal wastewater treatment plant in vaal, South Africa. Int J Environ Res Public Health. 2020;17(3):1096.
- Daughton CG. Cradle-to-Cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. Rationale for and avenues toward a green pharmacy. Environ Health Perspect. 2003;111(5): 757-74.
- Daughton CG, Ruhoy IS. Green pharmacy and pharmEcovigilance: prescribing and the planet. Expert Rev Clin Pharmacol. 2011;4(2):211-32.
- Kreisberg J. Greener pharmacy: proper medicine disposal protects the environment. Integrative Medicine. 2007; 6(4): 50-2.
- McArdell CS, Molnar E, Suter MJF, et al. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt valley watershed, Switzerland. Environ Sci Technol. 2003;37(24):5479-86.
- Mudgal S, De Toni A, Lockwood S, et al.. Study on the environmental risks of medicinal products (Doctoral dissertation, BIO Intelligence Service, 20-22 Villa Deshayes, 75014 Paris; European Commission). 2013.
- Toma A, Ofelia C. Green pharmacy – a narrative review. Clujul Medical. 2018; 91(4):391.
- Thomaidis NS, Asimakopoulos AG, Bletsou AA. Emerging contaminants: a tutorial mini-review. Global NEST Journal. 2012; 14(1):72-9.
- Lapworth DJ, Baran N, Stuart ME, et al. Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environmental pollution. 2012;163:287-303.
- Oulton RL, Kohn T, Cwiertny DM. Pharmaceuticals and personal care products in effluent matrices: a survey of transformation and removal during wastewater treatment and implications for wastewater management. Journal of Environmental Monitoring. 2010;12(11): 1956-78.
- Pal A, Gin KY, Lin AY, et al. Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Science of The Total Environment. 2010;408(24): 6062-9.
- Kansal A. Temporal and spatial assessment of water quality of major rivers of Uttarakhand: impact of sewage and industrial effluent (Doctoral dissertation, College of Engineering, University of Petroleum & Energy Studies); 2012.
- Dhangar K, Kumar M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: a review. Science of The Total Environment. 2020;738:140320.
- Courtheyn D, Le Bizec B, Brambilla G, et al. Recent developments in the use and abuse of growth promoters. Anal Chim Acta. 2002;473(1-2):71-82.
- Comber S, Gardner M, Sörme P, et al. Active pharmaceutical ingredients entering the aquatic environment from wastewater treatment works: a cause for concern?. Science of The Total Environment. 2018;613:538-47.
- Ternes T. Pharmaceuticals and metabolites as contaminants of the aquatic environment. In Pharmaceuticals and Care Products in the Environment ACS Symposium Series. Daughton (Washington, DC: American Chemical Society), 2001; 2–39.
- Kreisberg J. Ecological healing and the web of life. Explore (New York, NY). 2005;1(2):133-5.
- Kummerer K. Pharmaceuticals in the Environment: sources, fate, effects and risks. Springer; 2001.
- Cuthbert R, Parry-Jones J, Green RE, et al. NSAIDs and scavenging birds: potential impacts beyond Asia’s critically endangered vultures. Biol Lett. 2007;3(1):91-4.
- Gonzalez-Rey M, Bebianno MJ. Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquatic Toxicology. 2014;148: 221-30.
- Yan J, Lin W, Gao Z, et al. Use of selected NSAIDs in Guangzhou and other cities in the world as identified by wastewater analysis. Chemosphere. 2021;279:130529.
- Rodarte-Morales AI, Feijoo G, Moreira MT, et al. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete Chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation. 2012;23(1):145-56.
- Ortúzar M, Esterhuizen M, Olicón-Hernández DR, et al. Pharmaceutical pollution in aquatic environments: a concise review of environmental impacts and bioremediation systems. Front Microbiol. 2022;13: 869332.
- Hirsch R, Ternes T, Haberer K, et al. Occurrence of antibiotics in the aquatic environment. Science of The Total Environment. 1999;225(1-2):109-18.
- Halling-Sørensen B, Nors Nielsen S, Lanzky PF, et al. Occurrence, fate and effects of pharmaceutical substances in the environment- a review. Chemosphere. 1998;36(2):357-93.
- Costanzo SD, Murby J, Bates J. Ecosystem response to antibiotics entering the aquatic environment. Mar Pollut Bull. 2005;51(1-4):218-23.
- Larsson DGJ. Pollution from drug manufacturing: review and perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014; 369(1656): 20130571.
- Wang H, Wang N, Wang B, et al. Antibiotics detected in urines and adipogenesis in school children. Environ Int. 2016;89:204-11.
- Zafar R, Bashir S, Nabi D, et al. Occurrence and quantification of prevalent antibiotics in wastewater samples from Rawalpindi and Islamabad, Pakistan. Science of The Total Environment. 2021;764: 142596.
- Zainab SM, Junaid M, Xu N, et al. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: a global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020;187:116455.
- Kidd KA, Blanchfield PJ, Mills KH, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences. 2007;104(21):8897-901.
- Mutiyar PK, Gupta SK, Mittal AK. Fate of pharmaceutical active compounds (PhACs) from River Yamuna, India: an ecotoxicological risk assessment approach. Ecotoxicol Environ Saf. 2018; 150:297-304.
- Sharma M, Chaudhry S. Impact of industrial pollution on Yamuna river: a review. Kurukshetra, India : ResearchGate Publication. 2015.
- Sayadi MH, Trivedy RK, Pathak RK. Pollution of pharmaceuticals in the environment. Journal of Industrial Pollution Control. 2010;26(1):89-94.
- Yu X, Hu X, Li S, et al. Attitudes and practice regarding disposal for unwanted medications among young adults and elderly people in China from an ecopharmacovigilance perspective. Int J Environ Res Public Health. 2019;16(8):1463.
- Bondarczuk K, Piotrowska-Seget Z. Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Science of The Total Environment. 2019;650:2951-61.
- Wu S, Zhang L, Chen J. Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol. 2012;96(4):875-84.
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary. InAntimicrobial resistance global report on surveillance: 2014 summary .2014. Available from: https://www. who.int/ publications/i/item/ WHO-HSE-PED-AIP-2014.2. [cited Jun 30, 2014].
- Gore AC, Crews D, Doan LL, et al. Introduction to Endocrine Disrupting Chemicals (EDCs). a guide for public interest organizations and policy-makers. Washington, 2014:21–2.
- Manivel, Ranganathan R. Prioritized ABC FSN analysis of inventory management in private and hospital pharmacy followed by questionnaire. International Research Journal of Pharmacy. 2016;7(12):111-3.
- Yi M, Sheng Q, Sui Q, et al. β-blockers in the environment: distribution, transformation, and ecotoxicity. Environmental Pollution. 2020;266: 115269.
- Ulvi A, Aydın S, Aydın ME. Fate of selected pharmaceuticals in hospital and municipal wastewater effluent: occurrence, removal, and environmental risk assessment. Environ Sci Pollut Res Int. 2022;29(50):75609-25.
- Kudu I, Pillay V, Moodley B. Antiretrovirals (ARVs) in the environment. Emerging Freshwater Pollutants. Elsevier; 2022:227-39.
- Santos LH, Gros M, Rodriguez-Mozaz S, et al. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Science of The Total Environment. 2013; 461: 302-16.
- Neri NA, Velazquez AE, Islas-Flores H, et al. Pharmaceuticals and other pollutants as solid waste from hospitals and their toxic effects on aquatic organisms. Environmental Science and Engineering, JN Govil, Ed., Houston, TX: Studium Press LLC. 2017; 6: 171-201.
- Verlicchi P, Al Aukidy M, Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment--a review. Science of The Total Environment. 2012; 429:123-55.
- Jones OA, Voulvoulis N, Lester JN. Human pharmaceuticals in the aquatic environment a review. Environ Technol. 2001;22(12):1383-94.
- Pérez S, Barceló D, Aga D. Advances in the analysis of pharmaceuticals in the aquatic environment. Fate of Pharmaceuticals
in the Environment and in Water Treatment Systems. CRC Press; 2007:53-80. - Heath E, Filipič M, Kosjek T, et al. Fate and effects of the residues of anticancer drugs in the environment. Environ Sci Pollut Res Int. 2016;23(15):14687-91.
- da Costa Araújo AP, Mesak C, Montalvão MF, et al. Anti-cancer drugs in aquatic environment can cause cancer: insight about mutagenicity in tadpoles. Science of The Total Environment. 2019;650:2284-93.
- Aquino SF, Brandt EMF, Bottrel SEC, et al. Occurrence of pharmaceuticals and endocrine disrupting compounds in Brazilian water and the risks they may represent to human health. Int J Environ Res Public Health. 2021;18(22):11765.
- Sodré FF, Pescara IC, Montagner CC, et al. Assessing selected estrogens and xenoestrogens in Brazilian surface waters by liquid chromatography–tandem mass spectrometry. Microchemical Journal. 2010;96(1):92-8.
- Moreira M, Aquino S, Coutrim M, et al. Determination of endocrine‐disrupting compounds in waters from Rio das Velhas, Brazil, by liquid chromatography/high resolution mass spectrometry (ESI‐LC‐IT‐TOF/MS). Environ Technol. 2011;32(12):1409-17.
- Gadipelly C, Pérez-González A, Yadav GD, et al. Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res. 2014;53(29):11571-92.
- Weber FA, aus der Beek T, Bergmann A, et al. Pharmaceuticals in the environment – the global perspective: occurrence, effects, and potential cooperative action under SAICM. German Federal Environmental Agency; 2016.
- Kapoor D. Impact of pharmaceutical industries on environment, health and safety. Journal Of Critical Reviews. 2015;2(4):25-30.
- Fatta D, Achilleos A, Nikolaou A, et al. Analytical methods for tracing pharmaceutical residues in water and wastewater. Trends Analyt Chem. 2007;26(6):515-33.
- Vumazonke S, Khamanga SM, Ngqwala NP. Detection of pharmaceutical residues in surface waters of the Eastern Cape Province. Int J Environ Res Public Health. 2020;17(11):4067.
- Pratyusha K, Gaikwad NM, Phatak AA, et al. Review on: waste material management in pharmaceutical industry. Int. J Pharm Sci Rev Res. 2012; 16(2): 121-9.
- Boroski M, Rodrigues AC, Garcia JC, et al. Combined electrocoagulation and TiO2 photoassisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J Hazard Mater. 2009;162(1):448-54.
- Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017: 37-46.
- de Campos EA, Ten Caten CS, de Paula IC. End-of-use and end-of-life medicines—insights from pharmaceutical care process into waste medicines management. Environ Sci Pollut Res Int. 2021; 28(41):58170-88.
- Devnani M, Gupta A, Nigah R. ABC and VED analysis of the pharmacy store of a tertiary care teaching, research and referral healthcare institute of India. J Young Pharm. 2010; 2(2): 201-5.
- Rogowska J, Zimmermann A. Household pharmaceutical waste disposal as a global problem-a review. Int J Environ Res Public Health. 2022;19(23): 15798.
- Jessen M. Industry product stewardship and beverage container waste management programs in Canada [Internet]. 2002. Available from: http:// www.zerowaste.co.nz/assets/Govsolutions/Jesson-IndustryStewardshipandProducerResponsibilityinCanada.pdf. [cited May 23, 2002].
- Gadipelly C, Pérez-González A, Yadav GD, et al. Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res. 2014;53(29):11571-92.
- Sapkota B, Pariatamby A. Pharmaceutical waste management system – are the current techniques sustainable, eco-friendly and circular? a review. Waste Management. 2023;168:83-97.
- Ortúzar M, Esterhuizen M, Olicón-Hernández DR, et al. Pharmaceutical pollution in aquatic environments: a concise review of environmental impacts and bioremediation systems. Front Microbiol. 2022;13: 869332.
- Bottoni P, Caroli S, Caracciolo AB. Pharmaceuticals as priority water contaminants. Toxicol Environ Chem. 2010;92(3):549-65.
- Freitas LD, Radis-Baptista G. Pharmaceutical pollution and disposal of expired, unused, and unwanted medicines in the Brazilian context. J Xenobiot. 2021;11(2):61-76.
- Desai M, Njoku A, Nimo-Sefah L. Comparing environmental policies to reduce pharmaceutical pollution and address disparities. Int J Environ Res Public Health. 2022;19(14):8292.
- Environmental Protection Agency. Environmental Justice [Internet]. Available from: https:// www.epa.gov/environmentaljustice [cited Apr 25, 2022].
- U.S. Department of Health and Human Services. Disparities [Internet]. Available from: https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities#6 [cited Mar 1, 2022].
- Medical Waste Report : U.S Environmental Protection Agency(EPA), 2022, Medical Waste Management Report, Available from: https://www.epa.gov/ medicalwaste. [cited Aug 20, 2022]; National Health Commission of the People's Republic of China, 2021, Available from: http://www.nhc.gov.cn/. [cited Jun 20, 2021]; India Ministry of Environment, Forest and Climate Change, 2020, Available from: https://cpcb.nic.in/. [cited Oct 24, 2020]; European Environment Agency(EEA), 2023, Available from: https:// www.eea.europa.eu/. [cited Sep 15, 2023]; Brazil Ministry of Health, 2019, Available from: https://www.gov.br/saude/. [cited Jul 25, 2019]; South Africa Department of health, 2021, Available from: https://www.environment.gov.za/. [cited Nov 14 2021].
- Sharma AK. Bio medical waste (management and handling) rules. Bhopal: Suvidha Law House. 1998: 50-70.
- Pratyusha K, Gaikwad NM, Phatak AA, et al. Review on: waste material management in pharmaceutical industry. Int. J Pharm Sci Rev Res. 2012;16(2):121-9.
- Kimmell TA, Williams LR, Sorini SS. The RCRA Toxicity Characteristic Leaching Procedure (TCLP): a concept for a new method. Federal Facilities Environmental Journal. 2001;12(3):7-24.
- Siru D. Technical guidelines on healthcare waste management. NIPH.org.kh. 2011:1-65.
- Mostafa GM, Shazly MM, Sherief WI. Development of a waste management protocol based on assessment of knowledge and practice of healthcare personnel in surgical departments. Waste Management. 2009; 29(1):430-9.
- Wongiel S, Kumie A, Ashenef A. An assessment of pharmaceutical waste management by pharmaceutical industries and importers in and around Addis Ababa, Ethiopia. Ethiopian Journal of Environmental Studies & Management. 2018; 11(4).
- Rehman MS, Rashid N, Ashfaq M, et al. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere. 2015;138:1045-55.
- Aus der Beek T, Weber FA, Bergmann A, et al. Pharmaceuticals in the environment—global occurrences and perspectives. Environmental Toxicology and Chemistry. 2016;35(4):823-35.
- Hughes SR, Kay P, Brown LE. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol. 2013;47(2):661-77.
- Wilkinson JL, Boxall AB, Kolpin DW, et al. Pharmaceutical pollution of the world’s rivers. Proceedings of the National Academy of Sciences. 2022;119(8):e2113947119.
Type of Study: Systematic Review |
Subject:
Environmental pollution
Received: 2024/11/16 | Accepted: 2025/01/20 | Published: 2025/03/10
Received: 2024/11/16 | Accepted: 2025/01/20 | Published: 2025/03/10
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




