Volume 2, Issue 3 (September 2017)
J Environ Health Sustain Dev 2017, 2(3): 333-339 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dalvand A, Ehrampoush M H, Ghaneian M T, Mokhtari M, Ebrahimi A A, Malek Ahmadi R et al . Application of Chemical Coagulation Process for Direct Dye Removal from Textile Wastewater
. J Environ Health Sustain Dev 2017; 2 (3) :333-339
URL: http://jehsd.ssu.ac.ir/article-1-82-en.html
URL: http://jehsd.ssu.ac.ir/article-1-82-en.html
Arash Dalvand 
 , Mohammad Hassan Ehrampoush
, Mohammad Hassan Ehrampoush 
 , Mohammad Taghi Ghaneian
, Mohammad Taghi Ghaneian 
 , Mehdi Mokhtari
, Mehdi Mokhtari 
 , Ali Asghar Ebrahimi
, Ali Asghar Ebrahimi 
 , Roya Malek Ahmadi
, Roya Malek Ahmadi 
 , Amir Hossein Mahvi *
, Amir Hossein Mahvi * 


 , Mohammad Hassan Ehrampoush
, Mohammad Hassan Ehrampoush 
 , Mohammad Taghi Ghaneian
, Mohammad Taghi Ghaneian 
 , Mehdi Mokhtari
, Mehdi Mokhtari 
 , Ali Asghar Ebrahimi
, Ali Asghar Ebrahimi 
 , Roya Malek Ahmadi
, Roya Malek Ahmadi 
 , Amir Hossein Mahvi *
, Amir Hossein Mahvi * 

Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 563 kb]
(1380 Downloads)
| Abstract (HTML) (2714 Views)

Figure 4: The effect of the initial pH of wastewater on the dye removal efficiency and the final pH of wastewater (dye concentration: 50 mg/L, ferric chloride dose: 40 mg/L).
Full-Text: (1051 Views)
Application of Chemical Coagulation Process for Direct Dye Removal from Textile Wastewater
Arash Dalvand 1, Mohammad Hassan Ehrampoush 1, Mohammad Taghi Ghaneian 1, Mehdi Mokhtari 1,
Ali Asghar Ebrahimi 1, Roya Malek Ahmadi 1, Amir Hossein Mahvi 2,3,4*
1 Environmental Science and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
3 Center for Solid Waste Research, Institute for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran.
4 National Institute of Health Research, Tehran University of Medical Sciences, Tehran, Iran.
Arash Dalvand 1, Mohammad Hassan Ehrampoush 1, Mohammad Taghi Ghaneian 1, Mehdi Mokhtari 1,
Ali Asghar Ebrahimi 1, Roya Malek Ahmadi 1, Amir Hossein Mahvi 2,3,4*
1 Environmental Science and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
3 Center for Solid Waste Research, Institute for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran.
4 National Institute of Health Research, Tehran University of Medical Sciences, Tehran, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Since some dyes and their metabolites have toxicity and carcinogenicity potential, and are dangerous to the health of different living creatures, discharge of untreated wastewaters containing large concentrations of dye compounds into water resources is one of the important environmental problems. Therefore, in this research, the efficiency of chemical coagulation process was investigated using ferric chloride coagulant to remove Direct Red 23 dye from colored wastewater. Materials and Methods: In the experiments, a Jartest device was used and the effect of different parameters including the dose of the coagulant (20-200 mg/L), pH (3-10), and initial concentration of the dye (25-200 mg/L) on the efficiency of chemical coagulation process for removal of the dye was examined. Further, the final pH of the wastewater was investigated and the optimal conditions were determined. Results: The results indicated that the optimal dose of ferric chloride coagulant for Direct Red 23 dye removal of 97.7% is 40 mg/L at the optimal pH of 7. With increase in the dose of the coagulant, the dye removal efficiency increased, while the final pH of the wastewater decreased. Under constant conditions, with increase in the dye concentration, the dye removal efficiency diminished. Conclusion: Chemical coagulation by ferric chloride is a very effective and fast method for removal of direct dye from colored wastewater. |
|
| Article History: Received: 26 May 2017 Accepted: 10 August 2017 |
||
| *Corresponding Author: Amir Hossein Mahvi Email: ahmahvi@yahoo.com Tel: +982188651400 |
||
| Keywords: Dye, Removal, Coagulation |
Citation: Dalvand A, Ehrampoush MH, Ghaneian MT, et al. Application of chemical coagulation process for direct dye removal from textile wastewater. J Environ Health Sustain Dev. 2017; 2(3): 333-9.
Introduction
Textile industries produce large amounts of colored wastewater due to the consumption of large amounts of dye chemical compounds and high quality water 1. Since some dyes and their metabolites are mutagenic, carcinogenic and potentially dangerous to the health of living creatures, the discharge of wastewaters with a high concentration of dye compounds into the environment is considered as one of the important environmental problems 2. Due to the low biodegradability of dye compounds, physicochemical methods including coagulation and flocculation, electrochemical treatment 3, adsorption, oxidation, advanced oxidation, photo catalysis, and membrane methods are usually used to treat colored wastewaters 4. Among the mentioned methods, coagulation is the most widely used technique with a high efficiency for treatment of colored wastewaters, especially wastewaters containing soluble dyes 5. In the chemical coagulation method, coagulants based on iron or aluminum salts 6 such as alum, ferric chloride and ferric sulfate are usually used for the removal of contaminants from water and wastewater. In recent years, various studies were conducted on usage of coagulation and flocculation process for the removal of contaminants including dye 7, organic compounds 8 and turbidity 9 from wastewater, with results suggesting suitable efficiency of the coagulation method in the removal of the contaminants. Direct dyes are a group of anion dyes which are completely water-soluble 10. Direct Red 23 dye, which is used in textile industries for dying flax fiber, is a carcinogenic dye, and conventional treatment methods (e.g. biological methods) are not able to remove it effectively from wastewater 11. According to the studies, so far, no research has been conducted on usage of chemical coagulation method using ferric chloride to remove the above mentioned dye from the wastewater of textile industries. Therefore, in this study, the efficiency of chemical coagulation method by ferric chloride coagulant for the removal of Direct Red 23 dye from colored wastewater was investigated. Further, the effect of parameters including the coagulant dose, initial pH of the wastewater and initial concentration of the dye on the efficiency of chemical coagulation process was examined, followed by investigation of the final pH and determination of the optimal conditions.
Materials and Methods
This study is an experimental research at the laboratory scale. In this research, to synthesize a synthetic wastewater, Direct Red 23 dye (Ciba Co.) was used. This dye has the chemical formula, C35H25N7Na2O10S2 and a molecular weight of 813.7 g/mol. Figure 1 shows the chemical structure of the dye. Ferric chloride (FeCl3.6H2O), sodium hydroxide (NaOH) and hydrogen chloride (HCl) were purchased from Merck Co., Germany. The stock solution, 2% of ferric chloride, was prepared by adding 2 g of the coagulant to 100 ml of distilled water. HCl and NaOH 0.1 M were used to adjust the wastewater pH. Jartest device was used to perform the coagulation experiments. For this purpose, first, every 1-L container was filled with 500 ml of synthetic wastewater containing a certain concentration of the dye. Next, a certain dose of the coagulant was added to each container using a pipette and the mixture was stirred for 2 min at 100 rpm (rapid stirring), and then the rate of the stirrer was reduced to 40 rpm (slow stirring), with this stage lasting for 20 min. Following the slow stirring, the formed flocs were allowed 90 min to precipitate and eventually, samples were taken from the surface of the supernatant and the concentration of the residual dye was analyzed. Each experiment was triplicated and the mean value of the results was presented. The dye concentration was determined by measuring the maximum absorption wavelength of the dye (wavelength of 505 nm) using spectrophotometer DR 5000. The dye removal percentage was determined by Equation 1:
η = [(C0 – C)/C0] × 100 (1)
Where, η is the dye removal efficiency (%); C0 and C represent the concentration of the dye (mg/L) before and after the chemical coagulation process, respectively.
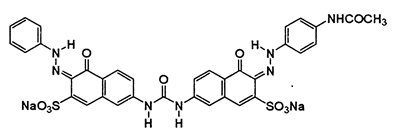
Figure 1: The chemical structure of the Direct Red 23 dye
Textile industries produce large amounts of colored wastewater due to the consumption of large amounts of dye chemical compounds and high quality water 1. Since some dyes and their metabolites are mutagenic, carcinogenic and potentially dangerous to the health of living creatures, the discharge of wastewaters with a high concentration of dye compounds into the environment is considered as one of the important environmental problems 2. Due to the low biodegradability of dye compounds, physicochemical methods including coagulation and flocculation, electrochemical treatment 3, adsorption, oxidation, advanced oxidation, photo catalysis, and membrane methods are usually used to treat colored wastewaters 4. Among the mentioned methods, coagulation is the most widely used technique with a high efficiency for treatment of colored wastewaters, especially wastewaters containing soluble dyes 5. In the chemical coagulation method, coagulants based on iron or aluminum salts 6 such as alum, ferric chloride and ferric sulfate are usually used for the removal of contaminants from water and wastewater. In recent years, various studies were conducted on usage of coagulation and flocculation process for the removal of contaminants including dye 7, organic compounds 8 and turbidity 9 from wastewater, with results suggesting suitable efficiency of the coagulation method in the removal of the contaminants. Direct dyes are a group of anion dyes which are completely water-soluble 10. Direct Red 23 dye, which is used in textile industries for dying flax fiber, is a carcinogenic dye, and conventional treatment methods (e.g. biological methods) are not able to remove it effectively from wastewater 11. According to the studies, so far, no research has been conducted on usage of chemical coagulation method using ferric chloride to remove the above mentioned dye from the wastewater of textile industries. Therefore, in this study, the efficiency of chemical coagulation method by ferric chloride coagulant for the removal of Direct Red 23 dye from colored wastewater was investigated. Further, the effect of parameters including the coagulant dose, initial pH of the wastewater and initial concentration of the dye on the efficiency of chemical coagulation process was examined, followed by investigation of the final pH and determination of the optimal conditions.
Materials and Methods
This study is an experimental research at the laboratory scale. In this research, to synthesize a synthetic wastewater, Direct Red 23 dye (Ciba Co.) was used. This dye has the chemical formula, C35H25N7Na2O10S2 and a molecular weight of 813.7 g/mol. Figure 1 shows the chemical structure of the dye. Ferric chloride (FeCl3.6H2O), sodium hydroxide (NaOH) and hydrogen chloride (HCl) were purchased from Merck Co., Germany. The stock solution, 2% of ferric chloride, was prepared by adding 2 g of the coagulant to 100 ml of distilled water. HCl and NaOH 0.1 M were used to adjust the wastewater pH. Jartest device was used to perform the coagulation experiments. For this purpose, first, every 1-L container was filled with 500 ml of synthetic wastewater containing a certain concentration of the dye. Next, a certain dose of the coagulant was added to each container using a pipette and the mixture was stirred for 2 min at 100 rpm (rapid stirring), and then the rate of the stirrer was reduced to 40 rpm (slow stirring), with this stage lasting for 20 min. Following the slow stirring, the formed flocs were allowed 90 min to precipitate and eventually, samples were taken from the surface of the supernatant and the concentration of the residual dye was analyzed. Each experiment was triplicated and the mean value of the results was presented. The dye concentration was determined by measuring the maximum absorption wavelength of the dye (wavelength of 505 nm) using spectrophotometer DR 5000. The dye removal percentage was determined by Equation 1:
η = [(C0 – C)/C0] × 100 (1)
Where, η is the dye removal efficiency (%); C0 and C represent the concentration of the dye (mg/L) before and after the chemical coagulation process, respectively.

Figure 1: The chemical structure of the Direct Red 23 dye
Results
The effect of coagulant dose on the dye removal efficiency and the final pH of wastewater
The effect of the dose of ferric chloride coagulant on the dye removal efficiency and the final pH of wastewater is shown in figure 2; This figure indicates that with increase in the dose of the coagulant, the dye removal efficiency increased, while the final pH of the wastewater diminished. Figure 3 shows the image of the dye solution before and after the coagulation process.

The effect of coagulant dose on the dye removal efficiency and the final pH of wastewater
The effect of the dose of ferric chloride coagulant on the dye removal efficiency and the final pH of wastewater is shown in figure 2; This figure indicates that with increase in the dose of the coagulant, the dye removal efficiency increased, while the final pH of the wastewater diminished. Figure 3 shows the image of the dye solution before and after the coagulation process.
Figure 2: The effect of coagulant dose on the dye removal efficiency and the final pH of wastewater (dye concentration: 50 mg/L, pH 7).

Figure 3: The image of dye solution before and after the coagulation (dye concentration: 50 mg/L, ferric chloride dose: 40 mg/L, pH 7)
Figure 3: The image of dye solution before and after the coagulation (dye concentration: 50 mg/L, ferric chloride dose: 40 mg/L, pH 7)
The effect of the initial pH of the wastewater on the dye removal efficiency and the final pH of wastewater
pH plays a very important role in the performance of chemical coagulation process for removal of contaminants from aqueous environments. Figure 4 shows the effect of the initial pH of the wastewater on the dye removal efficiency and final pH of the wastewater. As shown in the figure, considering ferric chloride with the optimal dose of 40 mg/L, the lowest dye removal efficiency was obtained at pH of 3 and 10, while the maximum removal efficiency was achieved at pH 7.
pH plays a very important role in the performance of chemical coagulation process for removal of contaminants from aqueous environments. Figure 4 shows the effect of the initial pH of the wastewater on the dye removal efficiency and final pH of the wastewater. As shown in the figure, considering ferric chloride with the optimal dose of 40 mg/L, the lowest dye removal efficiency was obtained at pH of 3 and 10, while the maximum removal efficiency was achieved at pH 7.
Figure 4: The effect of the initial pH of wastewater on the dye removal efficiency and the final pH of wastewater (dye concentration: 50 mg/L, ferric chloride dose: 40 mg/L).
The effect of the initial concentration of the dye on the dye removal efficiency
In order to determine the effect of the initial dye concentration on the dye removal efficiency during the chemical coagulation process under optimal conditions (pH 7, ferric chloride dose: 40 mg/L), four initial concentrations (25, 50, 100, and 200 mg/L) of dye were chosen. The results in figure 5 show that when the dye concentration increased from 25 to 200 mg/L, the dye removal efficiency reduced from 98.38 to 34%. The results in figure 5 suggest that even with increase in the dye concentration, the dye removal efficiency diminished and the quantity (milligram) of the removed dye increased per every milligram of the coagulant.


Figure 5: (a) The effect of the initial dye concentration on the dye removal efficiency, (b) mg of the removed dye per milligram of the coagulant (ferric chloride dose: 40 mg/L, pH 7).
In order to determine the effect of the initial dye concentration on the dye removal efficiency during the chemical coagulation process under optimal conditions (pH 7, ferric chloride dose: 40 mg/L), four initial concentrations (25, 50, 100, and 200 mg/L) of dye were chosen. The results in figure 5 show that when the dye concentration increased from 25 to 200 mg/L, the dye removal efficiency reduced from 98.38 to 34%. The results in figure 5 suggest that even with increase in the dye concentration, the dye removal efficiency diminished and the quantity (milligram) of the removed dye increased per every milligram of the coagulant.
Figure 5: (a) The effect of the initial dye concentration on the dye removal efficiency, (b) mg of the removed dye per milligram of the coagulant (ferric chloride dose: 40 mg/L, pH 7).
Discussion
The effect of coagulant dose on the dye removal efficiency and the final pH of wastewater
Figure 2 indicates that with increase in the coagulant dose from 20 to 200 mg/L, the dye removal efficiency increased from 50 to 99.13%. A similar trend has been reported by Merzouk et al. for the removal of disperse red dye from colored wastewater 12. Considering the high efficiency of 97.7% for the dye removal at the dose of 40 mg/L for ferric chloride, and considering the economic aspect in production of less sludge at lower doses of the coagulant, this dose was chosen as the optimal dose of ferric chloride. When ferric chloride coagulant (with the dose of 40 mg/L) was used, the final pH of wastewater reduced from 7 to 3.5. This reduction in pH can be due to the fact that when ferric chloride is added to wastewater, it is first hydrolyzed, thereby producing Fe3+ and Cl- ions. Then, Fe3+ ions react with the alkaline agents present in the wastewater, thereby producing insoluble sediment of Fe(OH)3. In response to consumption of the hydroxide ions in the environment, alkalinity diminishes, and in turn, the pH of the wastewater also drops. As shown in figure 3, the addition of ferric chloride coagulant resulted in coagulation of the dye molecules. The observations suggested that the flocs produced by ferric chloride were heavy, and their sedimentation rate was high, though they were fragile.
The effect of initial pH on the dye removal efficiency and the final pH of wastewater
The lower efficiency of dye removal at high pHs (Figure 4) can be associated with the formation of a soluble hydroxide genera of Fe(OH)4- 13, with these soluble hydroxide genera being unable to form flocs and entrap dye molecules, which in turn results in diminished dye removal efficiency. When using ferric chloride at neutral pH, the dominant mechanism of dye removal is adsorption, neutralization of the negative charge of dye molecules, and entrapment of contaminant particles in the Fe(OH)3 hydroxide sediments produced. The low dye removal efficiency at pH 3 can be due to the fact that, although at low pH, absorption and charge neutralization mechanism can cause neutralization of negative charge of the dye molecules, considering the low optimal dose of ferric chloride, the extent of formation of flocs and the size of formed flocs are not large enough. This in turn leads to diminished dye removal efficiency. It is noteworthy that when the concentration of materials to be coagulated is low in an aqueous environment, a coagulant is used at high doses; coagulant materials can act as a kernel for formation of large and sedimentable flocs, thereby causing effective removal of the contaminant. As shown in figure 4, even at high initial pH of the wastewater, the addition of ferric chloride resulted in the consumption of alkalinity and pH reduced to below 5. According to Iranian Environmental Protection Agency Standards, the allowable pH for discharge of wastewater to surface waters ranges between 6.5 and 8.5, before discharge of wastewater (which has been treated by ferric chloride) into water resources, modification of pH is essential.
The effect of initial dye concentration on dye removal efficiency
The diminished dye removal efficiency with increase in the initial dye concentration, can be attributed to the dye removal mechanism during the chemical coagulation process. The most important mechanisms for dye removal during chemical coagulation by ferric chloride are absorption and neutralization of the dye molecules (Fe3+ ions produced during ferric chloride hydrolysis process, which is able to neutralize the negative charge of dye molecules), as well as entrapment of the contaminant particles in the iron hydroxide sediments produced during the coagulation process 14. A constant dose of a coagulant is able to neutralize a certain amount of dye molecules. Therefore, at a constant dose of the coagulant, with increase in the initial concentration of the dye, the amount of the coagulant present for neutralization of the charge of all dye molecules is not large enough, and the removal efficiency decreases. On the other hand, with increase in the initial concentration of the dye and the constant dose of the coagulant, the chance of entrapment of dye molecules in the formed flocs increases. As a result, the quantity (milligram) of dye removed per every milligram of the coagulant increases.
Conclusion
Chemical coagulation process by ferric chloride coagulant can remove Direct Red 23 dye by as much as 97.7% under optimal conditions of pH 7, ferric chloride dose 40 mg/L. The results of the experiments indicated that the dye removal efficiency is in direct relationship with the coagulant dose, while it has an inverse relationship with the initial dye concentration. Furthermore, with increase in the wastewater pH (from 7 to 10), the dye removal efficiency drops. The results suggest that the usage of ferric chloride results in a dramatic decrease in wastewater pH. Therefore, before discharging wastewater treated with ferric chloride into the environment, the final pH should be modified in order to achieve wastewater discharge standards. Overall, the results of this research indicated that chemical coagulation treatment method using ferric chloride is effective and fast methods for removing direct dye from colored wastewater.
Acknowledgements
The authors would like to thank Tehran University of Medical Sciences for technical support of this Research.
Funding
The work was unfunded.
Conflict of interest
The authors declare no competing interests.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use.
References
1. Dalvand A, Gholami M, Joneidi A, et al. Dye removal, energy consumption and operating cost of electrocoagulation of textile wastewater as a clean process. Clean (Weinh). 2011; 39(7):
665-72.
2. Moghaddam SS, Moghaddam MA, Arami M. Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant. J Environ Manage. 2011; 92(4): 1284-91.
3. Mahmoodi NM, Dalvand A. Treatment of colored textile wastewater containing acid dye using electrocoagulation process. Desalination Water Treat. 2013; 51(31-33): 5959-64.
4. Huang X, Gao B, Yue Q, et al. Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: The effect of solution pH. Sep Purif Technol. 2015; 154: 108-14.
5. Lau Y-Y, Wong Y-S, Teng T-T, et al. Coagulation-flocculation of azo dye acid orange 7 with green refined laterite soil. Chem Eng J. 2014; 246: 383-90.
6. Khouni I, Marrot B, Moulin P, et al. Decolourization of the reconstituted textile effluent by different process treatments: enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination. 2011; 268(1): 27-37.
7. Papić S, Koprivanac N, Božić AL. Removal of reactive dyes from wastewater using Fe (III) coagulant. Coloration Technology. 2000; 116(11): 352-8.
8. Jung A-V, Chanudet V, Ghanbaja J, et al. Coagulation of humic substances and dissolved organic matter with a ferric salt: An electron energy loss spectroscopy investigation. Water Res. 2005; 39(16): 3849-62.
9. Ehteshami M, Maghsoodi S, Yaghoobnia E. Optimum turbidity removal by coagulation/ flocculation methods from wastewaters of natural stone processing. Desalination Water Treat. 2016; 57(44): 20749-57.
10. Dalvand A, Gholibegloo E, Ganjali MR, et al. Comparison of moringa stenopetala seed extract as a clean coagulant with alum and moringa stenopetala-alum hybrid coagulant to remove direct dye from textile wastewater. Environ Sci Pollut Res Int. 2016; 23(16): 16396-405.
11. Konicki W, Pełech I, Mijowska E, et al. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes- Fe 3 C nanocomposite: kinetics, equilibrium and thermodynamics. Chem Eng J. 2012; 210: 87-95.
12. Merzouk B, Gourich B, Madani K, et al. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation: A comparative study. Desalination. 2011; 272(1): 246-53.
13. Mackenzie LD. Water and Wastewater Engineering Design: Principles and Practice. eBook: McGraw-Hill Inc. 2010.
14. Zhang X, Yang Z, Wang Y, et al. The removal efficiency and reaction mechanism of aluminum coagulant on organic functional groups-carboxyl and hydroxyl. Chem Eng J. 2012; 211: 186-94.
The effect of coagulant dose on the dye removal efficiency and the final pH of wastewater
Figure 2 indicates that with increase in the coagulant dose from 20 to 200 mg/L, the dye removal efficiency increased from 50 to 99.13%. A similar trend has been reported by Merzouk et al. for the removal of disperse red dye from colored wastewater 12. Considering the high efficiency of 97.7% for the dye removal at the dose of 40 mg/L for ferric chloride, and considering the economic aspect in production of less sludge at lower doses of the coagulant, this dose was chosen as the optimal dose of ferric chloride. When ferric chloride coagulant (with the dose of 40 mg/L) was used, the final pH of wastewater reduced from 7 to 3.5. This reduction in pH can be due to the fact that when ferric chloride is added to wastewater, it is first hydrolyzed, thereby producing Fe3+ and Cl- ions. Then, Fe3+ ions react with the alkaline agents present in the wastewater, thereby producing insoluble sediment of Fe(OH)3. In response to consumption of the hydroxide ions in the environment, alkalinity diminishes, and in turn, the pH of the wastewater also drops. As shown in figure 3, the addition of ferric chloride coagulant resulted in coagulation of the dye molecules. The observations suggested that the flocs produced by ferric chloride were heavy, and their sedimentation rate was high, though they were fragile.
The effect of initial pH on the dye removal efficiency and the final pH of wastewater
The lower efficiency of dye removal at high pHs (Figure 4) can be associated with the formation of a soluble hydroxide genera of Fe(OH)4- 13, with these soluble hydroxide genera being unable to form flocs and entrap dye molecules, which in turn results in diminished dye removal efficiency. When using ferric chloride at neutral pH, the dominant mechanism of dye removal is adsorption, neutralization of the negative charge of dye molecules, and entrapment of contaminant particles in the Fe(OH)3 hydroxide sediments produced. The low dye removal efficiency at pH 3 can be due to the fact that, although at low pH, absorption and charge neutralization mechanism can cause neutralization of negative charge of the dye molecules, considering the low optimal dose of ferric chloride, the extent of formation of flocs and the size of formed flocs are not large enough. This in turn leads to diminished dye removal efficiency. It is noteworthy that when the concentration of materials to be coagulated is low in an aqueous environment, a coagulant is used at high doses; coagulant materials can act as a kernel for formation of large and sedimentable flocs, thereby causing effective removal of the contaminant. As shown in figure 4, even at high initial pH of the wastewater, the addition of ferric chloride resulted in the consumption of alkalinity and pH reduced to below 5. According to Iranian Environmental Protection Agency Standards, the allowable pH for discharge of wastewater to surface waters ranges between 6.5 and 8.5, before discharge of wastewater (which has been treated by ferric chloride) into water resources, modification of pH is essential.
The effect of initial dye concentration on dye removal efficiency
The diminished dye removal efficiency with increase in the initial dye concentration, can be attributed to the dye removal mechanism during the chemical coagulation process. The most important mechanisms for dye removal during chemical coagulation by ferric chloride are absorption and neutralization of the dye molecules (Fe3+ ions produced during ferric chloride hydrolysis process, which is able to neutralize the negative charge of dye molecules), as well as entrapment of the contaminant particles in the iron hydroxide sediments produced during the coagulation process 14. A constant dose of a coagulant is able to neutralize a certain amount of dye molecules. Therefore, at a constant dose of the coagulant, with increase in the initial concentration of the dye, the amount of the coagulant present for neutralization of the charge of all dye molecules is not large enough, and the removal efficiency decreases. On the other hand, with increase in the initial concentration of the dye and the constant dose of the coagulant, the chance of entrapment of dye molecules in the formed flocs increases. As a result, the quantity (milligram) of dye removed per every milligram of the coagulant increases.
Conclusion
Chemical coagulation process by ferric chloride coagulant can remove Direct Red 23 dye by as much as 97.7% under optimal conditions of pH 7, ferric chloride dose 40 mg/L. The results of the experiments indicated that the dye removal efficiency is in direct relationship with the coagulant dose, while it has an inverse relationship with the initial dye concentration. Furthermore, with increase in the wastewater pH (from 7 to 10), the dye removal efficiency drops. The results suggest that the usage of ferric chloride results in a dramatic decrease in wastewater pH. Therefore, before discharging wastewater treated with ferric chloride into the environment, the final pH should be modified in order to achieve wastewater discharge standards. Overall, the results of this research indicated that chemical coagulation treatment method using ferric chloride is effective and fast methods for removing direct dye from colored wastewater.
Acknowledgements
The authors would like to thank Tehran University of Medical Sciences for technical support of this Research.
Funding
The work was unfunded.
Conflict of interest
The authors declare no competing interests.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use.
References
1. Dalvand A, Gholami M, Joneidi A, et al. Dye removal, energy consumption and operating cost of electrocoagulation of textile wastewater as a clean process. Clean (Weinh). 2011; 39(7):
665-72.
2. Moghaddam SS, Moghaddam MA, Arami M. Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant. J Environ Manage. 2011; 92(4): 1284-91.
3. Mahmoodi NM, Dalvand A. Treatment of colored textile wastewater containing acid dye using electrocoagulation process. Desalination Water Treat. 2013; 51(31-33): 5959-64.
4. Huang X, Gao B, Yue Q, et al. Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: The effect of solution pH. Sep Purif Technol. 2015; 154: 108-14.
5. Lau Y-Y, Wong Y-S, Teng T-T, et al. Coagulation-flocculation of azo dye acid orange 7 with green refined laterite soil. Chem Eng J. 2014; 246: 383-90.
6. Khouni I, Marrot B, Moulin P, et al. Decolourization of the reconstituted textile effluent by different process treatments: enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination. 2011; 268(1): 27-37.
7. Papić S, Koprivanac N, Božić AL. Removal of reactive dyes from wastewater using Fe (III) coagulant. Coloration Technology. 2000; 116(11): 352-8.
8. Jung A-V, Chanudet V, Ghanbaja J, et al. Coagulation of humic substances and dissolved organic matter with a ferric salt: An electron energy loss spectroscopy investigation. Water Res. 2005; 39(16): 3849-62.
9. Ehteshami M, Maghsoodi S, Yaghoobnia E. Optimum turbidity removal by coagulation/ flocculation methods from wastewaters of natural stone processing. Desalination Water Treat. 2016; 57(44): 20749-57.
10. Dalvand A, Gholibegloo E, Ganjali MR, et al. Comparison of moringa stenopetala seed extract as a clean coagulant with alum and moringa stenopetala-alum hybrid coagulant to remove direct dye from textile wastewater. Environ Sci Pollut Res Int. 2016; 23(16): 16396-405.
11. Konicki W, Pełech I, Mijowska E, et al. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes- Fe 3 C nanocomposite: kinetics, equilibrium and thermodynamics. Chem Eng J. 2012; 210: 87-95.
12. Merzouk B, Gourich B, Madani K, et al. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation: A comparative study. Desalination. 2011; 272(1): 246-53.
13. Mackenzie LD. Water and Wastewater Engineering Design: Principles and Practice. eBook: McGraw-Hill Inc. 2010.
14. Zhang X, Yang Z, Wang Y, et al. The removal efficiency and reaction mechanism of aluminum coagulant on organic functional groups-carboxyl and hydroxyl. Chem Eng J. 2012; 211: 186-94.
Type of Study: Original articles |
Subject:
Special
Received: 2017/05/26 | Accepted: 2017/08/10 | Published: 2017/09/20
Received: 2017/05/26 | Accepted: 2017/08/10 | Published: 2017/09/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




