Volume 6, Issue 3 (September 2021)
J Environ Health Sustain Dev 2021, 6(3): 1340-1356 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Omozemoje Aigberua A, Chibueze Izah S, Richard G. Hazard Analysis of Trace Metals in Muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from Okulu River, Rivers State, Nigeria. J Environ Health Sustain Dev 2021; 6 (3) :1340-1356
URL: http://jehsd.ssu.ac.ir/article-1-376-en.html
URL: http://jehsd.ssu.ac.ir/article-1-376-en.html
Department of Microbiology, Faculty of Science, Bayelsa Medical University, Yenagoa, Bayelsa State, Nigeria.
Keywords: Aquatic Ecosystem, Environmental Health, Food Fish, Risk Assessment, Surface Water Contamination, Nigeria.
Full-Text [PDF 805 kb]
(481 Downloads)
| Abstract (HTML) (1600 Views)
.PNG)
Figure 1: Hierarchical cluster analysis of trace metals in different samples of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
TT – Tilapia (Sarotherodon melanotheron), CT- silver catfish (Chrysichthys nigrodigitatus )
.PNG)
Figure 2: Hierarchical cluster analysis of trace metals in Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu River, Rivers State, Nigeria
References
Full-Text: (560 Views)
Hazard Analysis of Trace Metals in Muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from Okulu River, Rivers State, Nigeria
Ayobami Omozemoje Aigberua 1, Sylvester Chibueze Izah 2*, Glory Richard 3
1 Department of Environment, Research and Development, Anal Concept Limited, Elelenwo, Rivers State, Nigeria.
2 Department of Microbiology, Faculty of Science, Bayelsa Medical University, Yenagoa, Bayelsa State, Nigeria.
3Department of Community Medicine, Faculty of Clinical Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria.
Ayobami Omozemoje Aigberua 1, Sylvester Chibueze Izah 2*, Glory Richard 3
1 Department of Environment, Research and Development, Anal Concept Limited, Elelenwo, Rivers State, Nigeria.
2 Department of Microbiology, Faculty of Science, Bayelsa Medical University, Yenagoa, Bayelsa State, Nigeria.
3Department of Community Medicine, Faculty of Clinical Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: An instance of fish deaths in marine waters surrounding some communities in Bonny and Andoni Local Government Areas in Rivers State was reported in March-April 2020. This study investigated trace metals hazard in muscle of Tilapia (Sarotherodon melanotheron) and Silver Catfish (Chrysichthys nigrodigitatus) from Okulu River, Rivers State, Nigeria. Materials and Methods: Tissues of 24 samples of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from the river were analyzed using atomic adsorption spectrophotometer, and the health risk was estimated based on estimated daily intake (EDI), target hazard quotient (THQ), and total target hazard quotient (TTHQ). Results: The concentration (mg/kg) of iron, zinc, manganese, copper, cadmium, lead, and chromium in both species ranged 4.00 – 197.30, 9.20 – 35.30, 0.20 – 5.00, 0.00 – 73.10, 0.00 – 1.30, 0.00 – 54.70, and 0.00 – 0.50, respectively. The EDI of trace metals resulting from the consumption of both fish species was higher than the permissible tolerance intake (PTI) mg/day/60kg body weight. The THQ and TTHQ were < 1, indicating that the consumption of this food fish portends no known health concern. However, the carcinogenic risks exceeded the threshold level of 10-6 - 10-4, thus, reflecting carcinogenic tendency. Based on the results obtained, it must be ensured that the effluents discharged into open water bodies meet the recommended limits. Conclusion: There is a need to create awareness among consumers of food fish in the study area. There should be periodic monitoring of trace metals in surface waters and its food fish population to forestall potential health impact on humans. |
|
| Article History: Received: 13 May 2021 Accepted: 20 July 2021 |
||
| *Corresponding Author: Sylvester Chibueze Izah Email: chivestizah@gmail.com Tel: + 2347030192466 |
||
| Keywords: Aquatic Ecosystem, Environmental Health, Food Fish, Risk Assessment, Surface Water Contamination, Nigeria. |
Citation: Aigberua AO, Izah SC, Richard G. Hazard Analysis of Trace Metals in Muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from Okulu River, Rivers State, Nigeria. J Environ Health Sustain Dev. 2021; 6(3): 1340-56.
Introduction
Waters are mainly classified as ground and surface waters (including brackish or estuarine, fresh, and marine environments). The various surface water bodies serve as habitat for diversities of both fin fishes and shellfishes, which are important sources of animal protein in human diet.
Trace metals, such as iron, lead1,2, copper, cadmium, manganese, chromium, zinc, nickel, and cobalt have recently emerged as common surface water micro-contaminants in the Niger Delta3. Human activities are the major source of surface water contamination. For instance, there have been several cases of crude oil spills on land and surface water environment. Furthermore, exacerbated concentration of trace metals tends to exterminate flora and fauna, posing health risks to humans.
Trace metals can be classified as potentially toxic metals, including cadmium, lead, nickel, chromium, and mercury 4. Trace metals can also be classified as essential (such as iron, copper, zinc, chromium, manganese, etc.) and non-essential (cadmium, lead, arsenic, mercury, etc.)5,6. Non-essential metals do not aid direct biological functions. On the other hand, even small amounts of essential heavy metals can be vital for the maintenance of cellular functions, enzymatic activities and other biological processes7,8. Trace metals aid in biosynthesis of glucose tolerance factor (chromium), activation of some enzymes and metabolism of cholesterol and mucopolysaccharide (manganese), cell and iron metabolism, and bone development (copper), among others6,8.
Fishes are known to bioaccumulate toxicants, such as trace metals in their tissues and organs. Several studies have been carried out with respect to determining trace metal levels in fish from different surface water resources in the Niger Delta, and particularly in Delta9, Bayelsa10,11, and Rivers State12. Most studies in the area compared metal ion concentrations with regulatory standard limits. However, studies have also indicated that the guideline limits alone do not sufficiently categorize the toxicity of trace metals, neither does it show the overall hazard index13,14.
The concept of risk assessment has been widely used to estimate the fate of toxicants on the environment (air, soil, and water) and foods, as well as discover its potential effects on human exposed to divergent contaminant sources8,13,15,16. Human or health risk is an important method of estimating the effect of toxicants, such as heavy metals. Human assimilate heavy metals via different routes, such as ingestion, inhalation, and dermal contact13,14,17. For trace metals in edible foods, such as fish, ingestion is the main exposure route.
Health risk is often estimated based on carcinogenic and non-carcinogenic threats. The common indices used to determine the non-carcinogenic risks include estimated daily intake (EDI), target hazard quotient (THQ), and hazard quotient (HQ)12,13,16,17,18. Also, carcinogenic risk can be determined for toxic metals, such as nickel, cadmium, arsenic, lead, chromium, etc.12,13,16,17.
Okulu River is an important water resource in the Niger Delta region of Nigeria. This is because it is bounded by several industries, whilst being utilized for artisanal dredging, boating and fishing activities, among others. The water is characterized by high conductivity, chloride and salinity, an indication that the environment is brackish in nature. Treated industrial effluents are discharged into surrounding rivers in the area. In 2020, there were reported instances of fish death in water resources that are linked to the Okulu River19. A reasonable stock of dead juvenile and average-sized croaker fish species were washed ashore the Oyorkotor and Finima beaches of Andoni and Bonny rivers, respectively19. Therefore, this study focused on [i] determining the concentration of trace metals in muscles of Tilapia (Sarotherodon melanotheron) and Silver Catfish (Chrysichthys nigrodigitatus), and comparing metal ion levels with stipulated guideline standards from different regulatory agencies; and [ii] estimating the health risk of trace metal in muscles of the two fish species obtained from Okulu River, Rivers State, Nigeria.
The findings from this study will provide information regarding the level of trace metals in tissues of the two species of fish. This information would be useful to residents of the area that consume food fish from the river. In addition, it will aid the estimation of potential health risks associated with their consumption, whilst serving as a guide for appropriate government agencies saddled with the responsibility of ensuring the absence of, or a healthy level of toxicants in
edible food. These regulatory agencies include European :union: (EU), Water Pollution
Control Legislation (WPCL), Food and
Agricultural Organization/World Health Organization (FAO/WHO), and United States Environmental Protection Agency (USEPA), etc.
Materials and method
Study area
Okulu River is an important River in the Niger Delta. The water is characterized by high conductivity (about 20,000 mg/L) and chloride (about 30,000 mg/L), an indication that the environment is a brackish/marine habitat. Treated industrial effluents, mainly depicting fresh water properties are discharged from a nearby petrochemical company into open water bodies. The section of river where fish was farmed is far from most industrial effluent discharge points. Other human activities characterizing this area include boating and transportation of goods and services. The climate of this area is similar to other parts of the Niger Delta as documented in the literature20,21.
Sample collection and handling
The fishes, Sarotherodon melanotheron and Chrysichthys nigrodigitatus were obtained with the aid of local fishermen in the area. Samples were randomly harvested, regardless of their sizes. As such, some samples were juvenile, while others were matured at the time of collection. Sampling was carried out during the rainy season month of July, 2020. Fishes were mainly harvested using gill nets, but some were caught using wounding gear (cutlass), whereby the fishermen stand along the shoreline to make incisions on straying fishes. A total of 24 fish samples were obtained, comprising of 12 samples each of both food fish species. The samples were labeled as TT1, TT2, TT3, ------TT12 and CT1, CT2, CT3, -----CT12 for Sarotherodon melanotheron and Chrysichthys nigrodigitatus, respectively. Fishes were caught with net traps after harvesting, and were immediately transported to the laboratory in a cooler packed with ice.
Sample processing and trace metals analysis
Fish samples were dissected, and the muscles isolated. Muscle tissues of food fish samples were oven-dried at 60oC for 24 hours, grinded and sieved, before being digested with an aqua-regia acid mixture of 69% nitric acid (Analar HNO3 – BDH Poole, United Kingdom) and 37% hydrochloric acid (Analar HCl – Sigma-Aldrich, Steinheim, Germany) in the ratio of 1:10 v/v, HNO3:HCl, respectively. The acid-extraction process was carried out in a fume cupboard. Afterwards, sample digests were filtered into volumetric flasks using 110 mm diameter Whatman filter paper no 1, before the filtration volume was made up to the mark with distilled water16, 22.
Instrument conditions and quality control measures adopted for trace metal analysis
The analytical procedures, working conditions of the flame atomic adsorption spectrophotometer (FAAS), and quality control protocols observed during analysis was similar to conditions previously described by Aigberua et al.22 and Izah and Aigberua16. The concentration of cadmium, chromium, lead, zinc, copper, iron, and manganese in acid-extracted samples were determined using FAAS (GBC 908 PBMT model, GBC Scientific - Australia). The working conditions of the instrument are provided in table 1. The quality control measures included reagent blank aspiration, replicate sampling, and analysis, as well as spike recovery. Percentage recovery was calculated based on Equation 1. Meanwhile, the spike recovery was carried out by mixing certified reference standards and test samples together. Subsequently, trace metals concentration was determined for the estimation of recoverable analyte concentration18. Reagent blanks (comprising extracting acid mixtures, but excluding sample introduction) were analyzed for metals of interest. The recovery values obtained ranged from 92.0% to 98.3% which is acceptable. For further quality assurance, the selected check standard solutions were analyzed after every batch of ten samples of fish extract. The limits of detection (LOD) and limits of quantification (LOQ) were both evaluated on the basis of standard deviation of absorbance response and the slope value of the calibration curve. Hence, the LOD, LOQ, and percentage spike recoveries of test metals are also presented in table1. The percentage recovery, LOD, and LOQ were calculated based on Equations 1, 2, and 3, respectively23,24.
% Recovery = [(metal concentration in spiked sample - metal concentration in unspiked sample)/(concentration of spike)] × 100 (1)
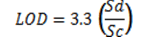 (2)
(2)
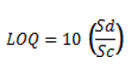 (3)
(3)
Where, Sd = standard deviation of the response
Sc = slope of calibration curve
Waters are mainly classified as ground and surface waters (including brackish or estuarine, fresh, and marine environments). The various surface water bodies serve as habitat for diversities of both fin fishes and shellfishes, which are important sources of animal protein in human diet.
Trace metals, such as iron, lead1,2, copper, cadmium, manganese, chromium, zinc, nickel, and cobalt have recently emerged as common surface water micro-contaminants in the Niger Delta3. Human activities are the major source of surface water contamination. For instance, there have been several cases of crude oil spills on land and surface water environment. Furthermore, exacerbated concentration of trace metals tends to exterminate flora and fauna, posing health risks to humans.
Trace metals can be classified as potentially toxic metals, including cadmium, lead, nickel, chromium, and mercury 4. Trace metals can also be classified as essential (such as iron, copper, zinc, chromium, manganese, etc.) and non-essential (cadmium, lead, arsenic, mercury, etc.)5,6. Non-essential metals do not aid direct biological functions. On the other hand, even small amounts of essential heavy metals can be vital for the maintenance of cellular functions, enzymatic activities and other biological processes7,8. Trace metals aid in biosynthesis of glucose tolerance factor (chromium), activation of some enzymes and metabolism of cholesterol and mucopolysaccharide (manganese), cell and iron metabolism, and bone development (copper), among others6,8.
Fishes are known to bioaccumulate toxicants, such as trace metals in their tissues and organs. Several studies have been carried out with respect to determining trace metal levels in fish from different surface water resources in the Niger Delta, and particularly in Delta9, Bayelsa10,11, and Rivers State12. Most studies in the area compared metal ion concentrations with regulatory standard limits. However, studies have also indicated that the guideline limits alone do not sufficiently categorize the toxicity of trace metals, neither does it show the overall hazard index13,14.
The concept of risk assessment has been widely used to estimate the fate of toxicants on the environment (air, soil, and water) and foods, as well as discover its potential effects on human exposed to divergent contaminant sources8,13,15,16. Human or health risk is an important method of estimating the effect of toxicants, such as heavy metals. Human assimilate heavy metals via different routes, such as ingestion, inhalation, and dermal contact13,14,17. For trace metals in edible foods, such as fish, ingestion is the main exposure route.
Health risk is often estimated based on carcinogenic and non-carcinogenic threats. The common indices used to determine the non-carcinogenic risks include estimated daily intake (EDI), target hazard quotient (THQ), and hazard quotient (HQ)12,13,16,17,18. Also, carcinogenic risk can be determined for toxic metals, such as nickel, cadmium, arsenic, lead, chromium, etc.12,13,16,17.
Okulu River is an important water resource in the Niger Delta region of Nigeria. This is because it is bounded by several industries, whilst being utilized for artisanal dredging, boating and fishing activities, among others. The water is characterized by high conductivity, chloride and salinity, an indication that the environment is brackish in nature. Treated industrial effluents are discharged into surrounding rivers in the area. In 2020, there were reported instances of fish death in water resources that are linked to the Okulu River19. A reasonable stock of dead juvenile and average-sized croaker fish species were washed ashore the Oyorkotor and Finima beaches of Andoni and Bonny rivers, respectively19. Therefore, this study focused on [i] determining the concentration of trace metals in muscles of Tilapia (Sarotherodon melanotheron) and Silver Catfish (Chrysichthys nigrodigitatus), and comparing metal ion levels with stipulated guideline standards from different regulatory agencies; and [ii] estimating the health risk of trace metal in muscles of the two fish species obtained from Okulu River, Rivers State, Nigeria.
The findings from this study will provide information regarding the level of trace metals in tissues of the two species of fish. This information would be useful to residents of the area that consume food fish from the river. In addition, it will aid the estimation of potential health risks associated with their consumption, whilst serving as a guide for appropriate government agencies saddled with the responsibility of ensuring the absence of, or a healthy level of toxicants in
edible food. These regulatory agencies include European :union: (EU), Water Pollution
Control Legislation (WPCL), Food and
Agricultural Organization/World Health Organization (FAO/WHO), and United States Environmental Protection Agency (USEPA), etc.
Materials and method
Study area
Okulu River is an important River in the Niger Delta. The water is characterized by high conductivity (about 20,000 mg/L) and chloride (about 30,000 mg/L), an indication that the environment is a brackish/marine habitat. Treated industrial effluents, mainly depicting fresh water properties are discharged from a nearby petrochemical company into open water bodies. The section of river where fish was farmed is far from most industrial effluent discharge points. Other human activities characterizing this area include boating and transportation of goods and services. The climate of this area is similar to other parts of the Niger Delta as documented in the literature20,21.
Sample collection and handling
The fishes, Sarotherodon melanotheron and Chrysichthys nigrodigitatus were obtained with the aid of local fishermen in the area. Samples were randomly harvested, regardless of their sizes. As such, some samples were juvenile, while others were matured at the time of collection. Sampling was carried out during the rainy season month of July, 2020. Fishes were mainly harvested using gill nets, but some were caught using wounding gear (cutlass), whereby the fishermen stand along the shoreline to make incisions on straying fishes. A total of 24 fish samples were obtained, comprising of 12 samples each of both food fish species. The samples were labeled as TT1, TT2, TT3, ------TT12 and CT1, CT2, CT3, -----CT12 for Sarotherodon melanotheron and Chrysichthys nigrodigitatus, respectively. Fishes were caught with net traps after harvesting, and were immediately transported to the laboratory in a cooler packed with ice.
Sample processing and trace metals analysis
Fish samples were dissected, and the muscles isolated. Muscle tissues of food fish samples were oven-dried at 60oC for 24 hours, grinded and sieved, before being digested with an aqua-regia acid mixture of 69% nitric acid (Analar HNO3 – BDH Poole, United Kingdom) and 37% hydrochloric acid (Analar HCl – Sigma-Aldrich, Steinheim, Germany) in the ratio of 1:10 v/v, HNO3:HCl, respectively. The acid-extraction process was carried out in a fume cupboard. Afterwards, sample digests were filtered into volumetric flasks using 110 mm diameter Whatman filter paper no 1, before the filtration volume was made up to the mark with distilled water16, 22.
Instrument conditions and quality control measures adopted for trace metal analysis
The analytical procedures, working conditions of the flame atomic adsorption spectrophotometer (FAAS), and quality control protocols observed during analysis was similar to conditions previously described by Aigberua et al.22 and Izah and Aigberua16. The concentration of cadmium, chromium, lead, zinc, copper, iron, and manganese in acid-extracted samples were determined using FAAS (GBC 908 PBMT model, GBC Scientific - Australia). The working conditions of the instrument are provided in table 1. The quality control measures included reagent blank aspiration, replicate sampling, and analysis, as well as spike recovery. Percentage recovery was calculated based on Equation 1. Meanwhile, the spike recovery was carried out by mixing certified reference standards and test samples together. Subsequently, trace metals concentration was determined for the estimation of recoverable analyte concentration18. Reagent blanks (comprising extracting acid mixtures, but excluding sample introduction) were analyzed for metals of interest. The recovery values obtained ranged from 92.0% to 98.3% which is acceptable. For further quality assurance, the selected check standard solutions were analyzed after every batch of ten samples of fish extract. The limits of detection (LOD) and limits of quantification (LOQ) were both evaluated on the basis of standard deviation of absorbance response and the slope value of the calibration curve. Hence, the LOD, LOQ, and percentage spike recoveries of test metals are also presented in table1. The percentage recovery, LOD, and LOQ were calculated based on Equations 1, 2, and 3, respectively23,24.
% Recovery = [(metal concentration in spiked sample - metal concentration in unspiked sample)/(concentration of spike)] × 100 (1)
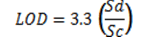 (2)
(2)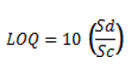 (3)
(3)Where, Sd = standard deviation of the response
Sc = slope of calibration curve
Table 1: Instrument working conditions and the applicable quality control parameters
| Test metals | Cu | Zn | Pb | Cr | Fe | Cd | Mn | |
| Slit width (nm) | 0.5 | 0.5 | 1 | 0.2 | 0.2 | 0.5 | 0.2 | |
| Wave-length(nm) | 324.7 | 213.9 | 217 | 357.9 | 248.3 | 228.8 | 279.5 | |
| Lamp Current (mA) | 3 | 5 | 5 | 6 | 7 | 3 | 5 | |
| Flame composition | Acetylene flow rate (L/min) | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| Air flow rate (L/min) | 11 | 11 | 11 | 11 | 11 | 11 | 11 | |
| Concentration of check standard (mg/L) | 5 | 0.5 | 2 | 1 | 1 | 0.5 | 2 | |
| LOD (mg/kg) | 0.004 | 0.005 | 0.008 | 0.016 | 0.013 | 0.0007 | 0.003 | |
| LOQ (mg/kg) | 0.012 | 0.015 | 0.024 | 0.048 | 0.039 | 0.002 | 0.009 | |
| Sample concentration (mg/kg) | 1.5 | 9.2 | 2 | 7.3 | 4 | 0.2 | 0.2 | |
| Spike amount (mg/kg) | 0.5 | 1 | 2 | 2 | 1 | 0.5 | 1 | |
| Determined amount (mg/kg) | 1.88 | 10.03 | 3.75 | 8.8 | 4.6 | 0.68 | 1.11 | |
| Percentage recovery (%) | 94 | 98.3 | 93.8 | 94.6 | 92 | 97.1 | 92.5 | |
Health risk assessment
Risk assessment has been widely used in estimating the potential health risk associated with trace metals. Several indices have been applied to determine the risks associated with trace elements in fish (carcinogenic and non-carcinogenic). These indices include estimated daily intake, target hazard quotient, total target hazard quotients, hazard quotients, and carcinogenic risks25. The description and resultant values used for the health risk assessment are presented in Table 2.
Estimated daily intake
Estimated daily intake was calculated based on the method previously described by Kortei et al.25, Yi et al.26, Bassey et al.27 and Okati et al.28. Detailed information about Equation 4 is shown in Table 2.
Estimated Daily Intake (EDI) = (4)
(4)
Target Hazard Quotients
Target Hazard Quotient was calculated based on the method previously described by Kortei et al.25 Chien et al.29 and Bassey et al.27. THQ is often used to show non-carcinogenic health effects associated with trace metals in ingestible materials, such as edible parts of fish. THQ < 1 indicates that exposure level is less than the RfD26. It also shows that the possibility of developing adverse health effects due to exposure is low or non-existent. Hence, daily ingestion of metals from food fish may not aggravate adverse health effects during a person's lifetime25,29. Conversely, when the value of THQ is greater than 1(> 1), it suggests that the exposure level is greater than the RfD, depicting potential adverse health effects. Detailed information about Equation 5 is shown in Table 2.
Target Hazard Quotients = (5)
(5)
Total target hazard quotient
Total target hazard quotient (TTHQ) is the sum of the individual trace metals. The TTHQ values was calculated following the method described by Kortei et al.25 Sadeghi et al.30 Chien et al.29 and Bassey et al.27.
TTHQ= (6)
(6)
Carcinogenic risk
The carcinogenic risk is often used to show the likelihood of an individual developing cancer due to exposure to potential carcinogens over a lifetime25. The carcinogenic risk of cadmium, lead, and chromium was calculated based on the method previously described by USEPA31 applied by Kortei et al.25.
Carcinogenic Risk = EDI CSF
CSF
Where CSF = Cancer Slope Factor, and EDI = Estimated daily intake
When the CR is <10-6, 10-6 - 10-4 and >10-4, it
indicates that it is “safe for human consumption”, “potential carcinogenic risks to the exposed population”, and “exposed population would encounter excess carcinogenic risk”, respectively32.
Risk assessment has been widely used in estimating the potential health risk associated with trace metals. Several indices have been applied to determine the risks associated with trace elements in fish (carcinogenic and non-carcinogenic). These indices include estimated daily intake, target hazard quotient, total target hazard quotients, hazard quotients, and carcinogenic risks25. The description and resultant values used for the health risk assessment are presented in Table 2.
Estimated daily intake
Estimated daily intake was calculated based on the method previously described by Kortei et al.25, Yi et al.26, Bassey et al.27 and Okati et al.28. Detailed information about Equation 4 is shown in Table 2.
Estimated Daily Intake (EDI) =
 (4)
(4)Target Hazard Quotients
Target Hazard Quotient was calculated based on the method previously described by Kortei et al.25 Chien et al.29 and Bassey et al.27. THQ is often used to show non-carcinogenic health effects associated with trace metals in ingestible materials, such as edible parts of fish. THQ < 1 indicates that exposure level is less than the RfD26. It also shows that the possibility of developing adverse health effects due to exposure is low or non-existent. Hence, daily ingestion of metals from food fish may not aggravate adverse health effects during a person's lifetime25,29. Conversely, when the value of THQ is greater than 1(> 1), it suggests that the exposure level is greater than the RfD, depicting potential adverse health effects. Detailed information about Equation 5 is shown in Table 2.
Target Hazard Quotients =
 (5)
(5)Total target hazard quotient
Total target hazard quotient (TTHQ) is the sum of the individual trace metals. The TTHQ values was calculated following the method described by Kortei et al.25 Sadeghi et al.30 Chien et al.29 and Bassey et al.27.
TTHQ=
 (6)
(6)Carcinogenic risk
The carcinogenic risk is often used to show the likelihood of an individual developing cancer due to exposure to potential carcinogens over a lifetime25. The carcinogenic risk of cadmium, lead, and chromium was calculated based on the method previously described by USEPA31 applied by Kortei et al.25.
Carcinogenic Risk = EDI
 CSF
CSFWhere CSF = Cancer Slope Factor, and EDI = Estimated daily intake
When the CR is <10-6, 10-6 - 10-4 and >10-4, it
indicates that it is “safe for human consumption”, “potential carcinogenic risks to the exposed population”, and “exposed population would encounter excess carcinogenic risk”, respectively32.
Table 2: Summary of abbreviation, units, and values used for the estimation of health risk assessment due to consumption of muscles of the fish species
| Factors | Abbreviations | Units | Values | Reference |
| Concentration of trace metals in fish | C | mg/kg | Current study | Current study |
| *Daily ingestion rate (based on per capita consumption) | IR | g/day/person | 36.44 | Current study |
| Body weight | BW | Kg | 60 for adult | 27 |
| Exposure frequency | EF | Day/year | 365 | 30,33 |
| Exposure duration | ED | Years | 48.9 | 27 |
| Conversion factor | - | Unit less | 3-10 | 30,33 |
| Average exposure time for non-carcinogens | ATn | Days/year | 365  ED ED |
27 |
| Average exposure time for carcinogens | ATc | Days/year | 365  70 70 |
25 |
| Oral reference dose | RFD | mg/kg/day | Cu = 0.04, Fe = 0.7, Zn = 0.3, Cr = 0.004, Mn = 0.14, Cd = 0.001, Pb = 1.5 |
16, 18,22, 27, 34 |
| Cancer slope factor | CSF | mg/kg/day | Cr = 0.5, | 32,35 |
| Pb = 0.0085 | 35,36 | |||
| Cd = 0.38 | 35,37 |
*The ingestion rate was calculated based on 13.3 kg/per/year presented by Bradley et al.38. Hence the values were multiplied by 1000 to convert to gram (g) and divided by 365 (number of days in a year).
Statistical analysis
Statistical Package for the Social Sciences version 20 was used to carry out the statistical analysis. T-test was used to compare the concentration of trace metals in both fish species. Significant difference was established at p < 0.05. Pearson correlation was used to show the significant relationship between the metals for each of the fish species. Hierarchical cluster analysis was done to show the distances between each fish species and the trace elements.
Ethical Issue
Ethical approval (REC/2021/0002) was obtained from the research and ethics committee of Bayelsa Medical University, Yenagoa, Bayelsa State, Nigeria.
Results
Concentration of trace metals in fish muscle
The level of trace metals in muscles of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu River, Rivers State, Nigeria is shown in table 3. Meanwhile, the statistical analysis of trace elements in muscles of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from the river is presented in table 4. The mean±standard error level of iron, zinc, manganese, copper, cadmium, lead, and chromium in the fishes were 21.39 ± 4.10 mg/kg, 21.58 ± 2.16 mg/kg, 2.10 ± 0.43 mg/kg, 40.46 ± 6.87 mg/kg, 0.48 ± 0.09 mg/kg, 19.97 ± 5.28 mg/kg, and 0.03 ± 0.03 mg/kg, respectively (Chrysichthys nigrodigitatus), and 53.00 ± 15.43 mg/kg, 19.38 ± 2.22 mg/kg, 2.62 ± 0.30 mg/kg, 5.31 ± 1.35 mg/kg, 0.55 ± 0.12 mg/kg, 16.65 ± 4.07 mg/kg, and 0.04 ± 0.04 mg/kg, respectively (Sarotherodon melanotheron). There was no significant difference (p > 0.05) between the concentration of Sarotherodon melanotheron and Chrysichthys nigrodigitatus for each of the trace metals, except for copper that showed a significant difference (p < 0.05).
Statistical Package for the Social Sciences version 20 was used to carry out the statistical analysis. T-test was used to compare the concentration of trace metals in both fish species. Significant difference was established at p < 0.05. Pearson correlation was used to show the significant relationship between the metals for each of the fish species. Hierarchical cluster analysis was done to show the distances between each fish species and the trace elements.
Ethical Issue
Ethical approval (REC/2021/0002) was obtained from the research and ethics committee of Bayelsa Medical University, Yenagoa, Bayelsa State, Nigeria.
Results
Concentration of trace metals in fish muscle
The level of trace metals in muscles of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu River, Rivers State, Nigeria is shown in table 3. Meanwhile, the statistical analysis of trace elements in muscles of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from the river is presented in table 4. The mean±standard error level of iron, zinc, manganese, copper, cadmium, lead, and chromium in the fishes were 21.39 ± 4.10 mg/kg, 21.58 ± 2.16 mg/kg, 2.10 ± 0.43 mg/kg, 40.46 ± 6.87 mg/kg, 0.48 ± 0.09 mg/kg, 19.97 ± 5.28 mg/kg, and 0.03 ± 0.03 mg/kg, respectively (Chrysichthys nigrodigitatus), and 53.00 ± 15.43 mg/kg, 19.38 ± 2.22 mg/kg, 2.62 ± 0.30 mg/kg, 5.31 ± 1.35 mg/kg, 0.55 ± 0.12 mg/kg, 16.65 ± 4.07 mg/kg, and 0.04 ± 0.04 mg/kg, respectively (Sarotherodon melanotheron). There was no significant difference (p > 0.05) between the concentration of Sarotherodon melanotheron and Chrysichthys nigrodigitatus for each of the trace metals, except for copper that showed a significant difference (p < 0.05).
Table 3: Level of trace metals (mg/kg) in Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
| Sample Code | Fe | Zn | Mn | Cu | Cd | Pb | Cr |
| CT1 | 4.00 | 9.20 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 |
| CT2 | 56.30 | 15.70 | 0.90 | 66.90 | 0.00 | 13.60 | 0.00 |
| CT3 | 9.40 | 32.50 | 2.60 | 15.90 | 1.20 | 34.30 | 0.00 |
| CT4 | 23.50 | 24.00 | 5.00 | 73.10 | 0.40 | 32.40 | 0.40 |
| CT5 | 20.90 | 30.20 | 2.40 | 46.10 | 0.70 | 37.00 | 0.00 |
| CT6 | 19.70 | 31.90 | 4.30 | 52.30 | 0.40 | 54.70 | 0.00 |
| CT7 | 15.40 | 20.50 | 1.50 | 55.60 | 0.40 | 31.40 | 0.00 |
| CT8 | 23.80 | 21.00 | 0.80 | 66.20 | 0.30 | 0.30 | 0.00 |
| CT9 | 11.80 | 17.40 | 0.60 | 45.80 | 0.70 | 0.00 | 0.00 |
| CT10 | 40.00 | 19.30 | 1.40 | 23.50 | 0.60 | 0.00 | 0.00 |
| CT11 | 13.50 | 24.90 | 3.00 | 18.30 | 0.50 | 12.50 | 0.00 |
| CT12 | 18.40 | 12.40 | 2.50 | 21.80 | 0.50 | 23.40 | 0.00 |
| TT1 | 33.60 | 23.40 | 4.00 | 0.00 | 0.80 | 39.50 | 0.00 |
| TT2 | 14.20 | 18.20 | 2.70 | 0.00 | 1.30 | 31.00 | 0.00 |
| TT3 | 106.80 | 17.30 | 4.00 | 4.30 | 0.90 | 29.40 | 0.00 |
| TT4 | 63.20 | 29.20 | 4.40 | 0.00 | 0.90 | 28.20 | 0.00 |
| TT5 | 197.30 | 35.30 | 2.10 | 10.80 | 0.60 | 26.20 | 0.50 |
| TT6 | 73.00 | 23.80 | 2.20 | 13.40 | 1.00 | 23.60 | 0.00 |
| TT7 | 44.30 | 13.60 | 1.70 | 9.20 | 0.40 | 4.00 | 0.00 |
| TT8 | 23.60 | 10.50 | 1.30 | 7.30 | 0.20 | 4.40 | 0.00 |
| TT9 | 21.70 | 21.00 | 2.30 | 0.70 | 0.00 | 2.10 | 0.00 |
| TT10 | 17.90 | 17.90 | 3.00 | 9.20 | 0.00 | 4.00 | 0.00 |
| TT11 | 29.00 | 14.60 | 1.60 | 2.40 | 0.40 | 2.60 | 0.00 |
| TT12 | 16.30 | 12.80 | 1.40 | 5.20 | 0.30 | 5.00 | 0.00 |
| *USEPA limit39, 40 | 0.50 | 5.00 | 0.02 | 2.25 | 0.01 | 0.11 | - |
| *WHO limit40,41 | 0.30 | 5.00 | 0.50 | 2.25 | 0.01 | 0.01 | - |
| *WPCL limit40,42 | 0.45 | 4.25 | 0.02 | 2.00 | 0.03 | 0.05 | - |
| FAO/WHO (Maximum allowable) limit43,44 | - | 40.00 | - | 30.00 | 0.50 | 0.50 | - |
| Median International Standard (Tolerable levels)45-47 | - | 45.00 | - | 20.00 | 0.30 | 2.00 | 1.00 |
| European :union: limit45,46,48 | - | - | - | - | 0.05 | 0.20 | - |
TT – Tilapia, Sarotherodon melanotheron and CT- silver catfish, Chrysichthys nigrodigitatus
* FAO/WHO-Food and Agricultural Organization/ World Health Organization; USEPA-United State Environmental Protection Agency; WPCL-Water Pollution Control Legislation, and WHO -World Health Organization.
Table 4: Comparative analysis of trace elements (mg/kg) in muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
* FAO/WHO-Food and Agricultural Organization/ World Health Organization; USEPA-United State Environmental Protection Agency; WPCL-Water Pollution Control Legislation, and WHO -World Health Organization.
Table 4: Comparative analysis of trace elements (mg/kg) in muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
| Parameters | Chrysichthys nigrodigitatus , n = 12 | Sarotherodon melanotheron, n = 12 | t-value | p-value |
| Fe | 21.39 ± 4.10 | 53.00 ± 15.43 | -1.979 | 0.060 |
| Zn | 21.58 ± 2.16 | 19.38 ± 2.22 | 0.714 | 0.483 |
| Mn | 2.10 ± 0.43 | 2.62 ± 0.30 | -0.986 | 0.335 |
| Cu | 40.46 ± 6.87 | 5.31 ± 1.35 | 5.023 | 0.000 |
| Cd | 0.48 ± 0.09 | 0.55 ± 0.12 | -0.485 | 0.632 |
| Pb | 19.97 ± 5.28 | 16.65 ± 4.07 | 0.498 | 0.624 |
| Cr | 0.03 ± 0.03 | 0.04 ± 0.04 | -0.156 | 0.877 |
Note: Data is expressed as mean±standard error
Correlation analysis
Table 5 shows the Pearson Correlation of heavy metals in muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria. Iron showed a positive relationship with chromium (r = 0.611, p < 0.01). Zinc showed a positive significant correlation with lead (r = 0.662, p < 0.01), cadmium (r = 0.468), and manganese (r = 0.485) at p < 0.05. A positive significant relationship was observed between manganese and cadmium with lead (r = 0.690) and lead (0.544) at p < 0.01, respectively.
Table 5 shows the Pearson Correlation of heavy metals in muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria. Iron showed a positive relationship with chromium (r = 0.611, p < 0.01). Zinc showed a positive significant correlation with lead (r = 0.662, p < 0.01), cadmium (r = 0.468), and manganese (r = 0.485) at p < 0.05. A positive significant relationship was observed between manganese and cadmium with lead (r = 0.690) and lead (0.544) at p < 0.01, respectively.
Table 5: Pearson correlation of trace elements in Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
| Parameters | Fe | Zn | Mn | Cu | Cd | Pb | Cr |
| Fe | 1.000 | ||||||
| Zn | 0.367 | 1.000 | |||||
| Mn | 0.127 | 0.485* | 1.000 | ||||
| Cu | -0.159 | 0.212 | -0.065 | 1.000 | |||
| Cd | 0.194 | 0.468* | 0.356 | -0.192 | 1.000 | ||
| Pb | 0.167 | 0.662** | 0.690** | 0.188 | 0.544** | 1.000 | |
| Cr | 0.611** | 0.400 | 0.246 | 0.194 | -0.001 | 0.203 | 1.000 |
**. Correlation is significant at the 0.01 level (2-tailed).
*. Correlation is significant at the 0.05 level (2-tailed).
*. Correlation is significant at the 0.05 level (2-tailed).
Cluster Analysis
Figures 1 and 2 show the hierarchical cluster analysis of trace metals based on the fish species. In the case of both fish species, two main clusters were formed viz: TTS in cluster 1, and all others in cluster 2. Cluster 1 formed sub-cluster. Within each sub-cluster, there were equal distances (Figure 1). Furthermore, in the case of trace elements, two major clusters were formed viz: iron in cluster 1, while all other trace elements were grouped within cluster 2. However, cluster 2 was further categorized into different sub-clusters (Figure 2).
Figures 1 and 2 show the hierarchical cluster analysis of trace metals based on the fish species. In the case of both fish species, two main clusters were formed viz: TTS in cluster 1, and all others in cluster 2. Cluster 1 formed sub-cluster. Within each sub-cluster, there were equal distances (Figure 1). Furthermore, in the case of trace elements, two major clusters were formed viz: iron in cluster 1, while all other trace elements were grouped within cluster 2. However, cluster 2 was further categorized into different sub-clusters (Figure 2).
.PNG)
Figure 1: Hierarchical cluster analysis of trace metals in different samples of Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu river, Rivers State, Nigeria
TT – Tilapia (Sarotherodon melanotheron), CT- silver catfish (Chrysichthys nigrodigitatus )
.PNG)
Figure 2: Hierarchical cluster analysis of trace metals in Sarotherodon melanotheron and Chrysichthys nigrodigitatus obtained from Okulu River, Rivers State, Nigeria
Health Risk Assessment
Estimated daily intake (mg/kg body weight/ day) of trace metals in the two fish species from Okulu River, Rivers State, Nigeria is shown in table 6. The EDI (mg/kg body weight/day) of iron, zinc, manganese, copper, cadmium, lead, and chromium in Chrysichthys nigrodigitatus and Sarotherodon melanotheron ranged from (1.30 – 19.50, 3.19 – 11.27, 0.07 – 1.73, 0.00 – 25.34, 0.00 – 0.21, 0.00 – 18.96 and 0.00 – 0.14), and (4.92 – 68.40, 3.64 – 12.24, 0.49 – 1.53, 0.00 – 4.65, 0.00 – 0.45, 0.73 – 13.69, and 0.00 – 0.17), respectively.
Table 7 shows the estimated THQ and TTHQ of trace metals in the two fish species from Okulu River, Rivers State, Nigeria. For the THQ, the values were 10-3 – 10-1 for iron, 10-2 for zinc, 10-4 – 10-2 for manganese, 10-2 – 10-1 for copper, 10-1 for cadmium, 10-1 for lead (except for TT1- TT6 and CT2- CT7, CT11, and CT12 with values >1.00), 10-4 for chromium in both fish species under study (Sarotherodon melanotheron and Chrysichthys nigrodigitatus). Basically, the THQ values for each of the metals were less than 1 (< 1) except for instances where the value was zero “0”. The TTHQ were > 1.00 in most of the fish samples (TT1- TT6 and CT2- CT7, CT11, and CT12), while the others were in the range of 10-2 – 10-1 for both food fish species.
Estimated carcinogenic risks of trace metals due to the consumption of the two fish species from the Okulu River, Rivers State, Nigeria are shown in table 8. Apart from CT1 and 2, and TT11 and 12, indicating values of zero (0), the carcinogenic risk value of cadmium was reportedly in the range of 10-2 – 10-1. For lead, the carcinogenic risk was in the range of 10-4 – 10-2 except for CT1, CT9, and CT10 that showed a value of zero (0). The chromium carcinogenic risk was in the order of 10-2 for CT4 and TT5, while the other sample codes revealed a value of zero “0”.
Estimated daily intake (mg/kg body weight/ day) of trace metals in the two fish species from Okulu River, Rivers State, Nigeria is shown in table 6. The EDI (mg/kg body weight/day) of iron, zinc, manganese, copper, cadmium, lead, and chromium in Chrysichthys nigrodigitatus and Sarotherodon melanotheron ranged from (1.30 – 19.50, 3.19 – 11.27, 0.07 – 1.73, 0.00 – 25.34, 0.00 – 0.21, 0.00 – 18.96 and 0.00 – 0.14), and (4.92 – 68.40, 3.64 – 12.24, 0.49 – 1.53, 0.00 – 4.65, 0.00 – 0.45, 0.73 – 13.69, and 0.00 – 0.17), respectively.
Table 7 shows the estimated THQ and TTHQ of trace metals in the two fish species from Okulu River, Rivers State, Nigeria. For the THQ, the values were 10-3 – 10-1 for iron, 10-2 for zinc, 10-4 – 10-2 for manganese, 10-2 – 10-1 for copper, 10-1 for cadmium, 10-1 for lead (except for TT1- TT6 and CT2- CT7, CT11, and CT12 with values >1.00), 10-4 for chromium in both fish species under study (Sarotherodon melanotheron and Chrysichthys nigrodigitatus). Basically, the THQ values for each of the metals were less than 1 (< 1) except for instances where the value was zero “0”. The TTHQ were > 1.00 in most of the fish samples (TT1- TT6 and CT2- CT7, CT11, and CT12), while the others were in the range of 10-2 – 10-1 for both food fish species.
Estimated carcinogenic risks of trace metals due to the consumption of the two fish species from the Okulu River, Rivers State, Nigeria are shown in table 8. Apart from CT1 and 2, and TT11 and 12, indicating values of zero (0), the carcinogenic risk value of cadmium was reportedly in the range of 10-2 – 10-1. For lead, the carcinogenic risk was in the range of 10-4 – 10-2 except for CT1, CT9, and CT10 that showed a value of zero (0). The chromium carcinogenic risk was in the order of 10-2 for CT4 and TT5, while the other sample codes revealed a value of zero “0”.
Table 6: Estimated daily intake (EDI) (mg/kg body weight/day) of trace metals due to the consumption of fish species from Okulu River, Rivers State, Nigeria
| Sample code | Fe | Zn | Mn | Cu | Cd | Pb | Cr |
| CT1 | 1.39 | 3.19 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| CT2 | 19.52 | 5.44 | 0.31 | 23.19 | 0.00 | 4.71 | 0.00 |
| CT3 | 3.26 | 11.27 | 0.90 | 5.51 | 0.42 | 11.89 | 0.00 |
| CT4 | 8.15 | 8.32 | 1.73 | 25.34 | 0.14 | 11.23 | 0.14 |
| CT5 | 7.25 | 10.47 | 0.83 | 15.98 | 0.24 | 12.83 | 0.00 |
| CT6 | 6.83 | 11.06 | 1.49 | 18.13 | 0.14 | 18.96 | 0.00 |
| CT7 | 5.34 | 7.11 | 0.52 | 19.27 | 0.14 | 10.89 | 0.00 |
| CT8 | 8.25 | 7.28 | 0.28 | 22.95 | 0.10 | 0.10 | 0.00 |
| CT9 | 4.09 | 6.03 | 0.21 | 15.88 | 0.24 | 0.00 | 0.00 |
| CT10 | 13.87 | 6.69 | 0.49 | 8.15 | 0.21 | 0.00 | 0.00 |
| CT11 | 4.68 | 8.63 | 1.04 | 6.34 | 0.17 | 4.33 | 0.00 |
| CT12 | 6.38 | 4.30 | 0.87 | 7.56 | 0.17 | 8.11 | 0.00 |
| TT1 | 11.65 | 8.11 | 1.39 | 0.00 | 0.28 | 13.69 | 0.00 |
| TT2 | 4.92 | 6.31 | 0.94 | 0.00 | 0.45 | 10.75 | 0.00 |
| TT3 | 37.02 | 6.00 | 1.39 | 1.49 | 0.31 | 10.19 | 0.00 |
| TT4 | 21.91 | 10.12 | 1.53 | 0.00 | 0.31 | 9.78 | 0.00 |
| TT5 | 68.40 | 12.24 | 0.73 | 3.74 | 0.21 | 9.08 | 0.17 |
| TT6 | 25.31 | 8.25 | 0.76 | 4.65 | 0.35 | 8.18 | 0.00 |
| TT7 | 15.36 | 4.71 | 0.59 | 3.19 | 0.14 | 1.39 | 0.00 |
| TT8 | 8.18 | 3.64 | 0.45 | 2.53 | 0.07 | 1.53 | 0.00 |
| TT9 | 7.52 | 7.28 | 0.80 | 0.24 | 0.00 | 0.73 | 0.00 |
| TT10 | 6.21 | 6.21 | 1.04 | 3.19 | 0.00 | 1.39 | 0.00 |
| TT11 | 10.05 | 5.06 | 0.55 | 0.83 | 0.14 | 0.90 | 0.00 |
| TT12 | 5.65 | 4.44 | 0.49 | 1.80 | 0.10 | 1.73 | 0.00 |
TT – Tilapia (Sarotherodon melanotheron), CT- silver catfish Chrysichthys nigrodigitatus )
Table 7: Estimated Target Hazard Quotient (THQ) and Total Target Hazard Quotient (TTHQ) of trace metals associated with the consumption of fish species from Okulu River, Rivers State, Nigeria
Table 7: Estimated Target Hazard Quotient (THQ) and Total Target Hazard Quotient (TTHQ) of trace metals associated with the consumption of fish species from Okulu River, Rivers State, Nigeria
| Sample code | THQ | TTHQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Zn | Mn | Cu | Cd | Pb | Cr | ||
| CT1 | 3.47E-03 | 1.86E-02 | 8.68E-04 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 2.30E-02 |
| CT2 | 4.88E-02 | 3.18E-02 | 3.90E-03 | 1.02E+00 | 0.00E+00 | 1.44E+00 | 0.00E+00 | 2.54E+00 |
| CT3 | 8.16E-03 | 6.58E-02 | 1.13E-02 | 2.41E-01 | 5.09E-01 | 3.64E+00 | 0.00E+00 | 4.47E+00 |
| CT4 | 2.04E-02 | 4.86E-02 | 2.17E-02 | 1.11E+00 | 1.70E-01 | 3.44E+00 | 1.13E-04 | 4.81E+00 |
| CT5 | 1.81E-02 | 6.11E-02 | 1.04E-02 | 7.00E-01 | 2.97E-01 | 3.92E+00 | 0.00E+00 | 5.01E+00 |
| CT6 | 1.71E-02 | 6.46E-02 | 1.87E-02 | 7.94E-01 | 1.70E-01 | 5.80E+00 | 0.00E+00 | 6.87E+00 |
| CT7 | 1.34E-02 | 4.15E-02 | 6.51E-03 | 8.44E-01 | 1.70E-01 | 3.33E+00 | 0.00E+00 | 4.41E+00 |
| CT8 | 2.06E-02 | 4.25E-02 | 3.47E-03 | 1.01E+00 | 1.27E-01 | 3.18E-02 | 0.00E+00 | 1.23E+00 |
| CT9 | 1.02E-02 | 3.52E-02 | 2.60E-03 | 6.95E-01 | 2.97E-01 | 0.00E+00 | 0.00E+00 | 1.04E+00 |
| CT10 | 3.47E-02 | 3.91E-02 | 6.07E-03 | 3.57E-01 | 2.55E-01 | 0.00E+00 | 0.00E+00 | 6.91E-01 |
| CT11 | 1.17E-02 | 5.04E-02 | 1.30E-02 | 2.78E-01 | 2.12E-01 | 1.33E+00 | 0.00E+00 | 1.89E+00 |
| CT12 | 1.60E-02 | 2.51E-02 | 1.08E-02 | 3.31E-01 | 2.12E-01 | 2.48E+00 | 0.00E+00 | 3.08E+00 |
| TT1 | 2.92E-02 | 4.74E-02 | 1.74E-02 | 0.00E+00 | 3.39E-01 | 4.19E+00 | 0.00E+00 | 4.62E+00 |
| TT2 | 1.23E-02 | 3.68E-02 | 1.17E-02 | 0.00E+00 | 5.52E-01 | 3.29E+00 | 0.00E+00 | 3.90E+00 |
| TT3 | 9.27E-02 | 3.50E-02 | 1.74E-02 | 6.53E-02 | 3.82E-01 | 3.12E+00 | 0.00E+00 | 3.71E+00 |
| TT4 | 5.48E-02 | 5.91E-02 | 1.91E-02 | 0.00E+00 | 3.82E-01 | 2.99E+00 | 0.00E+00 | 3.51E+00 |
| TT5 | 1.71E-01 | 7.15E-02 | 9.11E-03 | 1.64E-01 | 2.55E-01 | 2.78E+00 | 1.41E-04 | 3.45E+00 |
| TT6 | 6.33E-02 | 4.82E-02 | 9.54E-03 | 2.03E-01 | 4.24E-01 | 2.50E+00 | 0.00E+00 | 3.25E+00 |
| TT7 | 3.84E-02 | 2.75E-02 | 7.37E-03 | 1.40E-01 | 1.70E-01 | 4.24E-01 | 0.00E+00 | 8.07E-01 |
| TT8 | 2.05E-02 | 2.13E-02 | 5.64E-03 | 1.11E-01 | 8.49E-02 | 4.67E-01 | 0.00E+00 | 7.10E-01 |
| TT9 | 1.88E-02 | 4.25E-02 | 9.98E-03 | 1.06E-02 | 0.00E+00 | 2.23E-01 | 0.00E+00 | 3.05E-01 |
| TT10 | 1.55E-02 | 3.62E-02 | 1.30E-02 | 1.40E-01 | 0.00E+00 | 4.24E-01 | 0.00E+00 | 6.29E-01 |
| TT11 | 2.52E-02 | 2.96E-02 | 6.94E-03 | 3.64E-02 | 1.70E-01 | 2.76E-01 | 0.00E+00 | 5.44E-01 |
| TT12 | 1.41E-02 | 2.59E-02 | 6.07E-03 | 7.90E-02 | 1.27E-01 | 5.30E-01 | 0.00E+00 | 7.83E-01 |
TT – Tilapia (Sarotherodon melanotheron), CT- silver catfish (Chrysichthys nigrodigitatus )
Table 8: Estimated carcinogenic risks of trace metals due to the consumption of Tilapia and silver catfish species obtained from Okulu River, Rivers State, Nigeria
Table 8: Estimated carcinogenic risks of trace metals due to the consumption of Tilapia and silver catfish species obtained from Okulu River, Rivers State, Nigeria
| Sample Code | Cd | Pb | Cr |
| CT1 | 0.00E+00 | 0.00E+00 | 0.00E+00 |
| CT2 | 0.00E+00 | 1.23E-02 | 0.00E+00 |
| CT3 | 1.73E-01 | 3.09E-02 | 0.00E+00 |
| CT4 | 5.77E-02 | 2.92E-02 | 5.66E-05 |
| CT5 | 1.01E-01 | 3.34E-02 | 0.00E+00 |
| CT6 | 5.77E-02 | 4.93E-02 | 0.00E+00 |
| CT7 | 5.77E-02 | 2.83E-02 | 0.00E+00 |
| CT8 | 4.33E-02 | 2.70E-04 | 0.00E+00 |
| CT9 | 1.01E-01 | 0.00E+00 | 0.00E+00 |
| CT10 | 8.66E-02 | 0.00E+00 | 0.00E+00 |
| CT11 | 7.21E-02 | 1.13E-02 | 0.00E+00 |
| CT12 | 7.21E-02 | 2.11E-02 | 0.00E+00 |
| TT1 | 1.15E-01 | 3.56E-02 | 0.00E+00 |
| TT2 | 1.88E-01 | 2.79E-02 | 0.00E+00 |
| TT3 | 1.30E-01 | 2.65E-02 | 0.00E+00 |
| TT4 | 1.30E-01 | 2.54E-02 | 0.00E+00 |
| TT5 | 8.66E-02 | 2.36E-02 | 7.07E-05 |
| TT6 | 1.44E-01 | 2.13E-02 | 0.00E+00 |
| TT7 | 5.77E-02 | 3.61E-03 | 0.00E+00 |
| TT8 | 2.89E-02 | 3.97E-03 | 0.00E+00 |
| TT9 | 0.00E+00 | 1.89E-03 | 0.00E+00 |
| TT10 | 0.00E+00 | 3.61E-03 | 0.00E+00 |
| TT11 | 5.77E-02 | 2.34E-03 | 0.00E+00 |
| TT12 | 4.33E-02 | 4.51E-03 | 0.00E+00 |
TT – Tilapia (Sarotherodon melanotheron), CT- silver catfish (Chrysichthys nigrodigitatus )
Discussion
The concentration of trace elements in tissues of Tilapia (Sarotherodon melanotheron) and Silver catfish (Chrysichthys nigrodigitatus) showed the absence of significant variation (p > 0.05) between the two fish species, except for copper that showed significant difference (p < 0.05). Evidently, the species (Sarotherodon melanotheron) depicted higher trace metal bio-accumulation when compared with the Chrysichthys nigrodigitatus. This is likely due to relative maturation of the Sarotherodon melanotheron. Based on results from this study, iron, manganese, and chromium levels appeared to be lower than concentrations previously reported by Aigberua and Tarawou49 and Aigberua and Izah50 in tissues of Tilapia zilli and Tympanotonus fuscatus, respectively. Conversely, the current study depicted higher copper concentrations compared to results obtained by Aigberua and Tarawou49 and Aigberua and Izah50; while zinc and lead showed partial similarities with values recorded by both authors. The spike in trace metals concentration is probably due to agricultural run-offs, artisanal dredging activities, and possibly treated effluents discharged from the nearby petrochemical plant. Also the findings of cadmium and lead in this study were lower than the concentrations in muscles of Cithrinus citharus from Ikoli creek11 and Clarias camerunensis from Ikoli creek10. This suggested that Okulu River is more polluted compared to the rivers from which the Cithrinus citharus and Clarias camerunensis were obtained. It could also due to bioaccumulation level between different species.
Based on the Pearson correlation, most test metals did not reflect positive significant relationship, an indication that trace metals in the fish muscle emanated from multiple sources. Furthermore, close distances showed a significant relationship; while distant clusters showed the degree of dissociation13,21. The variation in some of the distances indicates differences in the bioaccumulation level by the food fish; while distance among trace metals depicts a disparity in sources. Some of the possible sources of heavy metals in water include agricultural run-offs, storm water drainages, waste dumpsite leachates, artisanal dredging activities, and make-shift refinery (oil bunkering activities)51,52.
The concentration of trace metals in fish tissues exceeded most of the permissible regulatory limits. For instance, iron concentration was higher than the 0.50 mg/kg limit specified by USEPA, WHO, and WPCL39,40, 0.30 mg/kg40,41, and 0.45 mg/kg40,42, respectively. Also, zinc values recorded in this study were higher than the values of 5.00 mg/kg, 5.00 mg/kg, and 4.25 mg/kg recommended by USEPA, WHO, and WPCL, respectively. However, these values were lower than the median international standard (MIS) tolerable level of 45.00 mg/kg45-47 and 40.00 mg/kg maximum allowable limit specified by Food and Agricultural organization/World Health Organization43,44.
The concentration of manganese in food fish also exceeded the regulatory limits of 0.02 mg/kg39,40, 0.50 mg/kg40,41, and 0.02 mg/kg40,42, by USEPA, WHO, and WPCL, respectively. Chromium concentration is lesser than the tolerable level of 45.00 mg/kg, stipulated by median international standard (MIS)45 - 47.
The level of copper recorded was higher than the regulatory limits of 2.25 mg/kg39,40, 2.25 mg/kg40,41 and 2.00 mg/kg40,42 specified by USEPA, WHO and WPCL, respectively. However, the MIS and FAO/WHO limits of 20.00 mg/kg45-47 and 30.00 mg/kg43,44 for copper were higher than the values recorded in Sarotherodon melanotheron, but lower than the concentration found in Chrysichthys nigrodigitatus. The observed concentration of cadmium was higher than the regulatory limit of 0.05 mg/kg, specified by European :union: (EU), also MIS tolerable level, USEPA, WHO and WPCL45,46,48 were 0.30 mg/kg45-47, 0.01 mg/kg39,40, 0.01 mg/kg40,41 and 0.03 mg/kg40,42, respectively, for both fish species. While only Sarotherodon melanotheron depicted mean values higher than the maximum allowable limit of 0.50 mg/kg specified by FAO/WHO43,44.
The concentration of lead in this study was higher than various regulatory limits of 0.5 mg/kg specified by FAO/WHO, also EU, MIS tolerable level, USEPA, WHO and WPCL43,44 were 0.20 mg/kg45,46,48, 2.00 mg/kg45-47, 0.11 mg/kg39,40, 0.01 mg/kg40,41 and 0.05 mg/kg40,42, respectively.
The concentration of trace metals in food fish from Okulu river is higher than previous levels recorded in muscles of catfish from Okumeshi river, Delta State53, Chrysicthys fuscatus from Calabar river, Cross Rivers State54, Synodentis clarias and Cithrinus citharus from River Nun, Bayelsa State11, as well as tissues of Oreochromics noloticus from Ikoli creek, Bayelsa State10, Polydactylus quadratifilis, Chrysicthys nigrodigitatus and Cynoglossus senegalensis purchased from Central market in Calabar, Nigeria27. Meanwhile, metal concentrations recorded in this study were similar to the values recorded in some fresh water fish from Warri River in Delta State6.
The variations observed in the results of the current study might be attributed to the differences in bioaccumulation level in the different fishes, probably owing to their biochemical compositions, and/or level of toxicants in the water body55. The authors have also linked similar disparities to variation in bioavailability of toxicants, intrinsic fish processes, trophic structure of the ecosystem, and variation in thresholds of toxicants55,56. According to Maurya and Malik57, toxicant bio-accumulation by fishes depends on their food sources, physiological state, and toxicological dynamics of the species.
Health risk assessment
Health risk associated with toxicants, such as trace metals was assessed in two groups, including carcinogenic and non-carcinogenic threats. Both are often used to show the potential adverse health effects associated with the toxicants at different exposure routes13. Basically, the toxicity associated with trace elements in the human body depends mainly on their daily intake. Daily intake also depends on the exposure routes, including inhalation, ingestion, and dermal. Among these exposure routes, ingestion is the commonest method through which trace elements enter the human body. The EDI of chromium was higher than the permissible tolerable intake (PTI) value of 0.20 mg/day/60kg body weight27. The EDI of cadmium and lead in this study were higher than the PTI values of 0.00035 mg/day/60kg body weight 27,58 and 0.0036 mg/day/60kg body weight27,59, respectively. The EDI of zinc recorded were less than the PTI value of 12 mg/day/60kg body weight 27,60, except for sample TT5 representing 4.17% of the total sample. The EDI of iron in this study was less than the PTI value of 12.50 mg/day/60kg body weight 27,60, except for the sample CT2 and CT10, TT3 – TT7, representing 29.17% of the total sample. The EDI of copper in Chrysichthys nigrodigitatus (CT2 – CT12) was higher than the PTI value of 5 mg/day27,61; while the value for Sarotherodon melanotheron (TT1 – TT12) was lower than the PTI value. High PTI value, particularly those exceeding metal guideline limits suggests possible health concern. The PTI values recorded in this study were higher than values reported in tissues of Polydactylus quadratifilis, Chrysicthys nigrodigitatus, and Cynoglossus senegalensis purchased from Central market in Calabar, Nigeria27.
THQ for each of the trace metals (cadmium, chromium, zinc, manganese, iron, and copper), as well as the TTHQ recorded values less than 1 (< 1). This suggests that there may be no known adverse health effect associated with these trace elements contained in muscle of the food fish. Furthermore, the THQ for lead exceeded 1.00 in most of the locations, an indication of potential health concern. The values of THQ and TTHQ recorded in this study were similar with values in Chrysichthys and Sarotherodon melanotheron collected from the Okrika estuary within the Niger Delta region, Nigeria9, Oreochromis niloticus and Clarias anguilaris from Ankobrah and Pra Rivers, Ghana25. In addition, the findings of the present study were in line with the study by Bassey et al.27 in which THQ and TTHQ were less than 1 (<1), indicating that trace metals stemming from the long-term consumption of selected fish species, including Polydactylus quadratifilis, Chrysicthys nigrodigitatus, and Cynoglossus senegalensis purchased from Calabar central market, Nigeria, posed no health risk.
Carcinogenic risk is often used to estimate the tendency that cancer could occur due to the consumption of either of the two food fish species. In this study, the estimated values of chromium, cadmium, and lead were higher than the regulatory permissible levels of 10−6-10−4. This suggests that there is a tendency of developing cancer during an individual’s life time, stemming from the consumption of muscles of food fish from Okulu River in Rivers State, Nigeria. The values were also higher than 1.57 E-07 recorded in Hypophthalmichthys molitrix consumed by the Sistan region, Iran4. This is an indication of different levels of trace element pollution in the waters the fishes were obtained.
Conclusions
The findings of this study indicated that the level of trace metals, includingcadmium, chromium, lead, copper, zinc, iron, and manganese in the edible muscle tissues of the fish species, Chrysichthys nigrodigitatus and Sarotherodon melanotheron exceeded the recommended levels specified by different regulatory agencies. The EDI of trace metals in both fish species surpassed the regulatory PTI for cadmium and lead, while it was less than the PTI of chromium. Furthermore, some of the PTI observed in this study was higher than permissible concentration. For the health implication, THQ of all detected trace metals and TTHQ (combined non-carcinogenic) of ingestible toxic metals from both fish species were less than 1 (<1). This indicates that there is no associated potential risk. Carcinogenic risk revealed trace metals (chromium, lead, and cadmium) loading that exceeded threshold value, indicating potential cancer risk to the exposed population. Hence, it is required to monitor trace elements in fish tissues from open water resources. Such assessment will help forestall the potential health risk associated with food fish consumption from the study area. Meanwhile, it would be vital for further studies to determine the physicochemical properties of the discharged effluent before incursion into the water body, as well as the metal loading of underlying sediment. This will help understand possible impact of the pollution point source, as well as the discovery of possible anthropogenic sources of metallic ions in aquatic biota. In addition, other non-essential elements, such as arsenic and mercury should be further assayed in food fish.
Acknowledgement
Thanks are owed to Anal Concept Limited, Port Harcourt, Nigeria, for supporting with the laboratory analysis of test samples.
Funding
The study did not receive any financial support from any establishment or agency.
Conflicts of interest
The authors declare no conflict of interest.
Authors Contributions
The lead author conceived the idea and participated in the field data/sample collection, and laboratory analysis. The second author carried out the statistical analysis, while all authors wrote the initial draft, and fine-tuned the final manuscript for approval.
Abbreviations
The concentration of trace elements in tissues of Tilapia (Sarotherodon melanotheron) and Silver catfish (Chrysichthys nigrodigitatus) showed the absence of significant variation (p > 0.05) between the two fish species, except for copper that showed significant difference (p < 0.05). Evidently, the species (Sarotherodon melanotheron) depicted higher trace metal bio-accumulation when compared with the Chrysichthys nigrodigitatus. This is likely due to relative maturation of the Sarotherodon melanotheron. Based on results from this study, iron, manganese, and chromium levels appeared to be lower than concentrations previously reported by Aigberua and Tarawou49 and Aigberua and Izah50 in tissues of Tilapia zilli and Tympanotonus fuscatus, respectively. Conversely, the current study depicted higher copper concentrations compared to results obtained by Aigberua and Tarawou49 and Aigberua and Izah50; while zinc and lead showed partial similarities with values recorded by both authors. The spike in trace metals concentration is probably due to agricultural run-offs, artisanal dredging activities, and possibly treated effluents discharged from the nearby petrochemical plant. Also the findings of cadmium and lead in this study were lower than the concentrations in muscles of Cithrinus citharus from Ikoli creek11 and Clarias camerunensis from Ikoli creek10. This suggested that Okulu River is more polluted compared to the rivers from which the Cithrinus citharus and Clarias camerunensis were obtained. It could also due to bioaccumulation level between different species.
Based on the Pearson correlation, most test metals did not reflect positive significant relationship, an indication that trace metals in the fish muscle emanated from multiple sources. Furthermore, close distances showed a significant relationship; while distant clusters showed the degree of dissociation13,21. The variation in some of the distances indicates differences in the bioaccumulation level by the food fish; while distance among trace metals depicts a disparity in sources. Some of the possible sources of heavy metals in water include agricultural run-offs, storm water drainages, waste dumpsite leachates, artisanal dredging activities, and make-shift refinery (oil bunkering activities)51,52.
The concentration of trace metals in fish tissues exceeded most of the permissible regulatory limits. For instance, iron concentration was higher than the 0.50 mg/kg limit specified by USEPA, WHO, and WPCL39,40, 0.30 mg/kg40,41, and 0.45 mg/kg40,42, respectively. Also, zinc values recorded in this study were higher than the values of 5.00 mg/kg, 5.00 mg/kg, and 4.25 mg/kg recommended by USEPA, WHO, and WPCL, respectively. However, these values were lower than the median international standard (MIS) tolerable level of 45.00 mg/kg45-47 and 40.00 mg/kg maximum allowable limit specified by Food and Agricultural organization/World Health Organization43,44.
The concentration of manganese in food fish also exceeded the regulatory limits of 0.02 mg/kg39,40, 0.50 mg/kg40,41, and 0.02 mg/kg40,42, by USEPA, WHO, and WPCL, respectively. Chromium concentration is lesser than the tolerable level of 45.00 mg/kg, stipulated by median international standard (MIS)45 - 47.
The level of copper recorded was higher than the regulatory limits of 2.25 mg/kg39,40, 2.25 mg/kg40,41 and 2.00 mg/kg40,42 specified by USEPA, WHO and WPCL, respectively. However, the MIS and FAO/WHO limits of 20.00 mg/kg45-47 and 30.00 mg/kg43,44 for copper were higher than the values recorded in Sarotherodon melanotheron, but lower than the concentration found in Chrysichthys nigrodigitatus. The observed concentration of cadmium was higher than the regulatory limit of 0.05 mg/kg, specified by European :union: (EU), also MIS tolerable level, USEPA, WHO and WPCL45,46,48 were 0.30 mg/kg45-47, 0.01 mg/kg39,40, 0.01 mg/kg40,41 and 0.03 mg/kg40,42, respectively, for both fish species. While only Sarotherodon melanotheron depicted mean values higher than the maximum allowable limit of 0.50 mg/kg specified by FAO/WHO43,44.
The concentration of lead in this study was higher than various regulatory limits of 0.5 mg/kg specified by FAO/WHO, also EU, MIS tolerable level, USEPA, WHO and WPCL43,44 were 0.20 mg/kg45,46,48, 2.00 mg/kg45-47, 0.11 mg/kg39,40, 0.01 mg/kg40,41 and 0.05 mg/kg40,42, respectively.
The concentration of trace metals in food fish from Okulu river is higher than previous levels recorded in muscles of catfish from Okumeshi river, Delta State53, Chrysicthys fuscatus from Calabar river, Cross Rivers State54, Synodentis clarias and Cithrinus citharus from River Nun, Bayelsa State11, as well as tissues of Oreochromics noloticus from Ikoli creek, Bayelsa State10, Polydactylus quadratifilis, Chrysicthys nigrodigitatus and Cynoglossus senegalensis purchased from Central market in Calabar, Nigeria27. Meanwhile, metal concentrations recorded in this study were similar to the values recorded in some fresh water fish from Warri River in Delta State6.
The variations observed in the results of the current study might be attributed to the differences in bioaccumulation level in the different fishes, probably owing to their biochemical compositions, and/or level of toxicants in the water body55. The authors have also linked similar disparities to variation in bioavailability of toxicants, intrinsic fish processes, trophic structure of the ecosystem, and variation in thresholds of toxicants55,56. According to Maurya and Malik57, toxicant bio-accumulation by fishes depends on their food sources, physiological state, and toxicological dynamics of the species.
Health risk assessment
Health risk associated with toxicants, such as trace metals was assessed in two groups, including carcinogenic and non-carcinogenic threats. Both are often used to show the potential adverse health effects associated with the toxicants at different exposure routes13. Basically, the toxicity associated with trace elements in the human body depends mainly on their daily intake. Daily intake also depends on the exposure routes, including inhalation, ingestion, and dermal. Among these exposure routes, ingestion is the commonest method through which trace elements enter the human body. The EDI of chromium was higher than the permissible tolerable intake (PTI) value of 0.20 mg/day/60kg body weight27. The EDI of cadmium and lead in this study were higher than the PTI values of 0.00035 mg/day/60kg body weight 27,58 and 0.0036 mg/day/60kg body weight27,59, respectively. The EDI of zinc recorded were less than the PTI value of 12 mg/day/60kg body weight 27,60, except for sample TT5 representing 4.17% of the total sample. The EDI of iron in this study was less than the PTI value of 12.50 mg/day/60kg body weight 27,60, except for the sample CT2 and CT10, TT3 – TT7, representing 29.17% of the total sample. The EDI of copper in Chrysichthys nigrodigitatus (CT2 – CT12) was higher than the PTI value of 5 mg/day27,61; while the value for Sarotherodon melanotheron (TT1 – TT12) was lower than the PTI value. High PTI value, particularly those exceeding metal guideline limits suggests possible health concern. The PTI values recorded in this study were higher than values reported in tissues of Polydactylus quadratifilis, Chrysicthys nigrodigitatus, and Cynoglossus senegalensis purchased from Central market in Calabar, Nigeria27.
THQ for each of the trace metals (cadmium, chromium, zinc, manganese, iron, and copper), as well as the TTHQ recorded values less than 1 (< 1). This suggests that there may be no known adverse health effect associated with these trace elements contained in muscle of the food fish. Furthermore, the THQ for lead exceeded 1.00 in most of the locations, an indication of potential health concern. The values of THQ and TTHQ recorded in this study were similar with values in Chrysichthys and Sarotherodon melanotheron collected from the Okrika estuary within the Niger Delta region, Nigeria9, Oreochromis niloticus and Clarias anguilaris from Ankobrah and Pra Rivers, Ghana25. In addition, the findings of the present study were in line with the study by Bassey et al.27 in which THQ and TTHQ were less than 1 (<1), indicating that trace metals stemming from the long-term consumption of selected fish species, including Polydactylus quadratifilis, Chrysicthys nigrodigitatus, and Cynoglossus senegalensis purchased from Calabar central market, Nigeria, posed no health risk.
Carcinogenic risk is often used to estimate the tendency that cancer could occur due to the consumption of either of the two food fish species. In this study, the estimated values of chromium, cadmium, and lead were higher than the regulatory permissible levels of 10−6-10−4. This suggests that there is a tendency of developing cancer during an individual’s life time, stemming from the consumption of muscles of food fish from Okulu River in Rivers State, Nigeria. The values were also higher than 1.57 E-07 recorded in Hypophthalmichthys molitrix consumed by the Sistan region, Iran4. This is an indication of different levels of trace element pollution in the waters the fishes were obtained.
Conclusions
The findings of this study indicated that the level of trace metals, includingcadmium, chromium, lead, copper, zinc, iron, and manganese in the edible muscle tissues of the fish species, Chrysichthys nigrodigitatus and Sarotherodon melanotheron exceeded the recommended levels specified by different regulatory agencies. The EDI of trace metals in both fish species surpassed the regulatory PTI for cadmium and lead, while it was less than the PTI of chromium. Furthermore, some of the PTI observed in this study was higher than permissible concentration. For the health implication, THQ of all detected trace metals and TTHQ (combined non-carcinogenic) of ingestible toxic metals from both fish species were less than 1 (<1). This indicates that there is no associated potential risk. Carcinogenic risk revealed trace metals (chromium, lead, and cadmium) loading that exceeded threshold value, indicating potential cancer risk to the exposed population. Hence, it is required to monitor trace elements in fish tissues from open water resources. Such assessment will help forestall the potential health risk associated with food fish consumption from the study area. Meanwhile, it would be vital for further studies to determine the physicochemical properties of the discharged effluent before incursion into the water body, as well as the metal loading of underlying sediment. This will help understand possible impact of the pollution point source, as well as the discovery of possible anthropogenic sources of metallic ions in aquatic biota. In addition, other non-essential elements, such as arsenic and mercury should be further assayed in food fish.
Acknowledgement
Thanks are owed to Anal Concept Limited, Port Harcourt, Nigeria, for supporting with the laboratory analysis of test samples.
Funding
The study did not receive any financial support from any establishment or agency.
Conflicts of interest
The authors declare no conflict of interest.
Authors Contributions
The lead author conceived the idea and participated in the field data/sample collection, and laboratory analysis. The second author carried out the statistical analysis, while all authors wrote the initial draft, and fine-tuned the final manuscript for approval.
Abbreviations
- Estimated daily intake (EDI)
- Permissible tolerance intake (PTI)
- Target hazard quotient (THQ)
- Total target hazard quotient (TTHQ)
- European :union: (EU)
- Water Pollution Control Legislation (WPCL)
- Food and Agricultural Organization/World Health Organization (FAO/WHO)
- United States Environmental Protection Agency (USEPA)
- Limits of detection (LOD)
- Limits of quantification (LOQ)
- Cancer Slope Factor (CSF)
- Oral Reference dose (RfD)
- Median international standard (MIS)
- World Health Organization (WHO)
- Flame atomic adsorption spectrophotometer (FAAS)
References
- Esmaeili A, Eslami H. Adsorption of Pb (II) and Zn (II) ions from aqueous solutions by Red Earth. MethodsX. 2020; 7: 100804.
- Esmaeili A, Eslami H. Efficient removal of Pb(II) and Zn(II) ions from aqueous solutions by adsorption onto a native natural bentonite. MethodsX. 2019; 6: 1979–85.
- Ekere NR, Yakubu NM, Ihedioha JN. Assessment of levels and potential health risk of heavy metals in water and selected fish species from the benue-niger river confluence, Lokoja, Nigeria. J Aquatic Food Prod Technol. 2018; 27(7); 772-82.
- Fakhri, Y, Djahed, B, Toolabi, A, et al. Potentially toxic elements (PTEs) in fillet tissue of common carp (Cyprinus carpio): a systematic review, meta-analysis and risk assessment study. Toxin Rev. 2020;40(3):1-12.
- Nabavi SMB, Hosseini M, Parsa Y, et al. Assessment of PCBs, heavy metals (Cd, Co, Ni, Pb), mercury and methyl mercury content in four fish commonly consumed in Iran. Toxicol Environ Health Sci. 2014; 6(2): 119-26
- Izah SC, Chakrabarty N, Srivastav AL. A review on heavy metal concentration in potable water sources in Nigeria: human health effects and mitigating measures. Expo Health. 2016; 8: 285–304.
- Maleki A, Azadi NA, Mansouri B, et al. Health risk assessment of trace elements in two fish species of Sanandaj Gheshlagh Reservoir, Iran. Toxicol Environ Health Sci. 2015; 7(1): 43-9
- Izah SC, Inyang IR, Angaye TCN, et al. A review of heavy metal concentration and potential health implications in beverages consumed in Nigeria. Toxics. 2017; 5(1): 1-15
- Aghoghovwia OA, Ohimain EI, Izah SC. Bioaccumulation of heavy metals in different tissues of some commercially important fish species from Warri River, Niger Delta, Nigeria. Biotechnol Res. 2016; 2(1): 25-32
- Ogamba EN, Izah SC, Ofoni-Ofoni AS. Bioaccumulation of chromium, lead and cadmium in the bones and tissues of oreochromis niloticus and clarias camerunensis from Ikoli creek, Niger Delta, Nigeria. Adv Sci J Zool. 2016; 1(1): 13–6.
- Ogamba EN, Izah SC, Ebiowe RG. Bioconcentration of Mercury, Lead and Cadmium in the bones and muscles of Citharinus citharus and Synodontis clarias from the Amassoma Axis of River Nun, Niger Delta, Nigeria. Res J Pharmacol Toxicol. 2015; 1(1): 21-3.
- Ogunola OS, Onada OA, Falaye AE. Ecological risk evaluation of biological and geochemical trace metals in Okrika Estuary. Inter J Environ Res. 2017; 11(2): 149–73.
- Ogamba EN, Charles EE, Izah SC. Distributions, pollution evaluation and health risk of selected heavy metal in surface water of Taylor creek, Bayelsa State, Nigeria. Toxicol Environ Health Sci. 2021; 13(2): 109–21.
- Mohammadi AA, Zae A, Majidi S, et al. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodX. 2019; 6: 1642–51.
- Uzoekwe SA, Izah SC, Aigberua AO. Environmental and human health risk of heavy metals in atmospheric particulate matter (PM10) around gas flaring vicinity in Bayelsa State, Nigeria. Toxicol Environ Health Sci. 2021. doi. /10.1007/s13530-021-00085-7.
- Izah SC, Aigberua AO. Microbial and heavy metal hazard analysis of edible tomatoes (Lycopersicon esculentum) in Port Harcourt, Nigeria. Toxicol Environ Health Sci. 2020; 12(4): 371–80.
- Rezeai H, Zaei A, Kamarehie B, et al. Levels, distributions and health risk assessment of lead, cadmium and arsenic found in drinking groundwater of Dehgolan’s villages, Iran. Toxicol Environ Health Sci. 2019; 11(1): 54–62.
- Miri M, Akbari E, Amrane A, et al. Health risk assessment of heavy metal intake due to fish consumption in the Sistan region, Iran. Environ Monit Assess. 2017; 189:583.
- Chinwo E. Thousands of dead fish washed up on rivers shoreline: government begins investigation, warns against consumption. Thisday Editorial. 2020.
- Izah SC, Angaye TCN, Ohimain EI. Climate change: some meteorological indicators and perception of farmers in Yenagoa metropolis, Bayelsa state, Nigeria. Inter J Geol Agric Environ Sci. 2015; 3(1): 56–60.
- Izah SC, Bassey SE, Ohimain EI. Assessment of heavy metal in cassava mill effluent contaminated soil in a rural community in the Niger Delta region of Nigeria. EC Pharmacol Toxicol. 2017; 4(5): 186-201.
- Aigberua AO, Izah SC, Isaac IU. Level and health risk assessment of heavy metals in selected seasonings and culinary condiments used in Nigeria. Biol Evid. 2018; 8(2): 6-15
- Carlson J, Wysoczanski A, Voigtman E. Limits of quantification–Yet another suggestion. Spectrochimica Acta Part B. 2014; 96: 69-73
- Aigberua AO. Composition of potential heavy metal contaminants in selected liquid and powdered herbal medicines commonly sold in Port Harcourt metropolis, Nigeria. International J Medicinal Plants Nat Prod. 2019; 5(1): 30-9
- Kortei NK, Heymann ME. Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra basins: Impact of illegal mining activities on food safety. Toxicol Rep. 2020; 7; 360–9
- Yi Y, Tang C, Yi T, et al. Health risk assessment of heavy metals in fish and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicol Environ Saf. 2017; 145: 295–302
- Bassey FI, Oguntunde FC, Iwegbue CMA, et al. Effects of processing on the proximate and metal contents in three fish species from Nigerian coastal waters. Food Sci Nutr. 2014 2(3): 272–81
- Okati N, Moghadam MS, Einollahipeer F. Mercury, arsenic and selenium concentrations in marine fish species from the Oman Sea, Iran, and health risk assessment. Toxicol Environ Health Sci. 2020; 13; 25–36
- Chien LC, Hung TC, Choang KY, et al. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ. 2020: 285: 177–85.
- Sadeghi P, Loghmani M, Frokhzad S. Human health risk assessment of heavy metals via consumption of commercial marine fish (Thunnusalbacares, Euthynnusaffinis, and Katsuwonuspelamis) in Oman Sea. Environ Sci Poll Res. 2020; 27: 14944–52.
- United States Environmental Protection Agency (USEPA). 2012 Edition of the Drinking Water Standards and Health Advisories, Office of Water. U.S. Environmental Protection Agency, Washington, DC, 2012.
- Huang XL, Qin DL, Gao L, et al. Distribution, contents and health risk assessment of heavy metal (loid)s in fish from different water bodies in Northeast China. Roy Soc Chem Adv. 2019; 9: 33130.
- United States Environmental Protection Agency (USEPA). Guidance for assessing chemical contaminant data for use in fish advisories, volume II. Risk assessment and fish consumption limits. (EPA 823-B-00-008). United States Environmental Protection Agency, Washington, DC, 2000.
- Iwegbue CMA, Bassey FI, Tesi GO, et al. Concentrations and health risk assessment of metals in chewing gums, peppermints and sweets in Nigeria. J Food Measur Character. 2015; 9: 160-74
- Gebeyehu HR, Bayissa LD. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS One. 2020; 15(1): e0227883.
- Kamunda C, Mathuthu M, Madhuku M. Health risk assessment of heavy metals in soils from witwatersrand gold mining basin, South Africa. Inter J Environ Res Public Health. 2016; 13: 663.
- Yang J, Ma S, Zhou J, et al. Heavy metal contamination in soils and vegetables and health risk assessment of inhabitants in Daye, China. J Inter Med Res. 2018; 46: 3374–87.
- Bradley B, Byrd KA, Atkins M, et al. Fish in food systems in Nigeria: A review. Penang, Malaysia: WorldFish. Program Report. Available from: http://www.fish.cgiar.org/ publications/fish-food-systems-nigeria-review. [Cited 21 July 2021]
- United States Environmental Protection Agency (USEPA). Quality criteria for water. office of water regulations standards, Washington DC, USA, 1986.
- Anim-Gyampo M, Kumi M, Zango MS. Heavy metals concentrations in some selected Fish Species in Tono Irrigation Reservoir in Navrongo, Ghana. J Environ Earth Sci. 2013; 3(1): 109-19.
- World Health Organization. Summary and conclusions of the 61st meeting of the joint FAO/WHO Expert Committee on Food Additives (JECFA). JECFA/61/sc, Rome, Italy, 2003.
- Water Pollution Control Legislation. Land based water quality classification. Official Journal, 25687, Water Pollution Control Legislation, Turkey, 2004.
- Elnabris KJ, Muzyed SK, El-Ashgar NM. Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J Association Arab Universit Basic Appl Sci. 2013; 13: 44–51.
- Food and Agricultural Organization/ World Health Organization. Evaluation of certain food additives and the contaminants mercury, lead and cadmium. WHO Technical Report, Series No. 505, 1989.
- Senarathne P, Pathiratne KAS, Pathlratne A. Heavy metal levels in food fish, Etroplus suratensis inhabiting Bolgoda Lake, Sri Lanka. Vidyodaya J Sci. 2006; 13: 115-26.
- Senarathne P, Pathiratne KAS. Accumulation of heavy metals in food fish, Mystusgulio inhabiting Bologoda Lake, Sri Lanka. Sri Lanka J Aquatic Sci. 2007; 12: 61-75.
- Philips DJH. Developing country aquaculture – trace chemical contaminants and public health concerns. In: Environment and Aquaculture in Developing Countries; Pullin RSV, Rosenthal H, Maclean JL, Eds ICLARl'V1 Conference Proceedings. 1993; 31: 296- 311.
- European :union:. The Commission of the European Communities, Commission regulation, (EC) No. 22112002 amending regulation (EC) No. 466/2001 setting maximum levels For certain contaminants in food stuff in order to protect public health. Official Journal of the European Communities, 2002.
- Aigberua A, Tarawou T. Assessment of heavy metals in muscle of Tilapia zilli from some Nun river estuaries in the Niger Delta region of Nigeria. Acad J Chem. 2017; 2(9): 96-101.
- Aigberua AO, Izah SC. Evaluation of heavy metals in tissue of Tympanotonus fuscatus sold in some markets in Port Harcourt metropolis, Nigeria. MOJ Toxicol. 2018; 4(5): 334-8.
- Aigberua A, Tarawou T. Water Quality Index (WQI) Assessment along Inland fresh waters of Taylor Creek in Bayelsa State, Nigeria. J Environ Treat Techniq. 2019; 7(3): 260-9.
- Aigberua A, Tarawou T, Erepamowei Y. Environmental assessment of imiringi river in south-south Nigeria: the water quality index approach. Curr World Environ. 2020; 15(1): 59-67.
- Ekeanyanwu CR, Ogbuinyi CA, Etienajirhevwe OF. Trace metals distribution in fish tissue, bottom sediments and water from Okumeshi river in Delta state, Nigeria. Environ Res J. 2011; 5(1): 6-10.
- Joseph AP, Eyo VO, Andem AB, et al. Assessment of some heavy metals in the tissues (gills, liver and muscle) of Clarias gariepinus from Calabar River, Cross River State, South-eastern Nigeria. J Coastal Life Med. 2016; 4(6): 430-4.
- Izah SC, Angaye TCN. Heavy metal concentration in fishes from surface water in Nigeria: Potential sources of pollutants and mitigation measures. Sky J Biochem Res. 2016; 5(4), 31-47
- Eneji IS, Shato R, Annune PA. Bioaccumulation of heavy metals in fish (tilapia zilli and clarias gariepinus) organs from river Benue, North Central Nigeria. Pak J Anal Environ Chem. 2011; 12(1 & 2): 25-31.
- Maurya PK, Malik DS. Bioaccumulation of heavy metals in tissues of selected fish species from Ganga river, India, and risk assessment for human health. Human Ecol Risk Assess: An Inter J. 2018; 25(4): 905-23.
- EFSA. Scientific opinion statement ion tolerable weekly intake for cadmium. EFSA J. 2011; 9:1975.
- World Health Organization. Malathion in drinking water. Background Document for Preparation of WHO Guidelines for Drinking Water Quality. World Health Organization. Geneva, 2003.
- National Research Council. Recommended dietary allowance.10th ed. National Academy Press, Washington D.C, 1989.
- World Health Organization. Guidelines for drinking water quality. 3rd ed. Geneva, 2008.
Type of Study: Original articles |
Subject:
Food safety and hygiene
Received: 2021/05/13 | Accepted: 2021/07/20 | Published: 2021/09/25
Received: 2021/05/13 | Accepted: 2021/07/20 | Published: 2021/09/25
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








