Volume 6, Issue 3 (September 2021)
J Environ Health Sustain Dev 2021, 6(3): 1367-1375 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dolatabadi M, Ghorbanian A, Ahmadzadeh S. Mg-Al–layered Double Hydroxide as Promising Sustainable Nanoadsorbent for Application in Water/Wastewater Treatment Processes; Diethyl Phthalate Removal. J Environ Health Sustain Dev 2021; 6 (3) :1367-1375
URL: http://jehsd.ssu.ac.ir/article-1-334-en.html
URL: http://jehsd.ssu.ac.ir/article-1-334-en.html
Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
Keywords: Diethyl Phthalate, Hydroxide, Mg-Al–Layered Double Sustainable Nanoadsorbent, Water/Wastewater Treatment.
Full-Text [PDF 972 kb]
(511 Downloads)
| Abstract (HTML) (1667 Views)
.PNG)
Figure 1: FE-SEM image of Mg–Al-LDH
.PNG)
Figure 2: The XRD pattern of Mg-Al-LDH
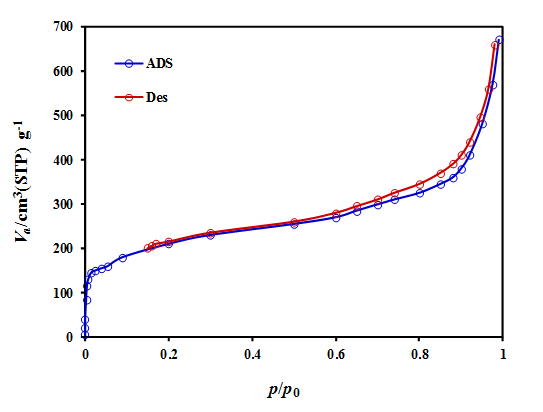
Figure 3: N2 adsorption-desorption isotherms of Mg-Al-LDH.

Figure 4: Effect of different DEP concentrations on the removal efficiency (LDH dosage of 0.15 g L-1, pH=7.5).
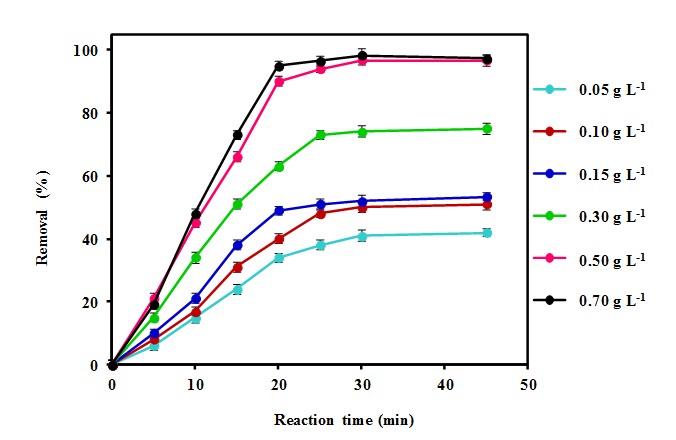
Figure 5: Effect of different adsorbent dosages (Mg–Al-LDH) on the removal efficiency
(DEP concentration 10 mg L-1, pH = 7.5).
Table 1: Parameters of first and second-order kinetics models for the adsorption of DEP using Mg-Al-LDH.
Full-Text: (508 Views)
Mg-Al–layered Double Hydroxide as Promising Sustainable Nanoadsorbent for Application in Water/Wastewater Treatment Processes; Diethyl Phthalate Removal
Maryam Dolatabadi 1, 2, Akram Ghorbanian 3, Saeid Ahmadzadeh 4, 5*
1 Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran.
2 Environmental Science and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3 Department of Environmental Health, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
4 Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
5 Pharmaceutical Sciences and Cosmetic Products Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Maryam Dolatabadi 1, 2, Akram Ghorbanian 3, Saeid Ahmadzadeh 4, 5*
1 Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran.
2 Environmental Science and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3 Department of Environmental Health, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
4 Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
5 Pharmaceutical Sciences and Cosmetic Products Research Center, Kerman University of Medical Sciences, Kerman, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Since phthalate esters and their derivatives have the potential to disrupt proper endocrine function, these compounds are considered as one of the most important groups of endocrine-disrupting chemicals. The presence of these compounds in various aquatic environments has caused main concerns about human and animal health and causes problems in the environment. Materials and Methods: The treatment process was carried out in a glass reactor containing 200 mL polluted water at room temperature. The Mg-Al layered double hydroxides (Mg-Al-LDH) were successfully synthesized and were applied as adsorbents for the removal of Diethyl Phthalate (DEP) from polluted water. The kinetics and isotherm of the process were investigated to determine the exact mechanism of DEP removal from the water medium. Results: The Mg-Al-LDH was a surface area of 673 (m2 g-1), a total pore of 0.716 (cm3 g-1), and microspore volumes of 0.627 (cm3 g-1), and a pore diameter of 8.64 nm. The maximum DEP removal efficiency of 96.7% was obtained at the DEP concentration of 10 mg L-1, Mg-Al-LDH dosage of 0.50 g L-1, and the reaction time of 30 min. The second-order kinetic model well depicted the kinetics of DEP adsorption (R2 = 0. 99). The Langmuir isotherm model best described the data by predicting the maximum adsorption capacity (qm) of 95.6 mg g-1 and R2 of 0.99. Conclusion: All the results demonstrate that the Mg-Al-LDH is an efficient, safe, and efficient adsorbent in water and wastewater treatment. |
|
| Article History: Received: 11May 2021 Accepted: 20 July 2021 |
||
| *Corresponding Author: Saeid Ahmadzadeh Email: chem_ahmadzadeh@yahoo.com Tel: +983431325241 |
||
| Keywords: Diethyl Phthalate, Hydroxide, Mg-Al–Layered Double Sustainable Nanoadsorbent, Water/Wastewater Treatment. |
Citation: Dolatabadi M, Ghorbanian A, Ahmadzadeh S. Mg-Al–layered Double Hydroxide as Promising Sustainable Nanoadsorbent for Application in Water/wastewater Treatment Processes; Diethyl Phthalate Removal. J Environ Health Sustain Dev. 2021; 6(3): 1367-75.
Introduction
Phthalates or phthalic acid esters (PAEs) are employed as common additives in paints and resins, printing inks, lubricants, floor coverings, adhesives, insecticides, packaging, cosmetic products, and as a solvent in the rubber and plastic industries. Some types of phthalate esters are already blacklisted as priority water pollutants by the Environmental Protection Agency (EPA) 1, 2. Phthalates are not chemically bound to plastics. Therefore, they exist as freely mobile and leachable compounds. They are released from soft plastics into the environment. Phthalates have been detected in various environments such as atmosphere, surface water, sediment, and sewage sludge at various concentrations in the range of ng L-1 to mg L-1 3, 4. The presence of phthalates and their derivatives in the environment causes many concerns about human and animal health. After these compounds enter into the human and animal body, they can accumulate and concentrate in their unchanged form or metabolized form, which are detected in the intracellular or extracellular body fluids such as blood, urine, milk, etc. Since phthalates have the potential to disrupt proper endocrine function, these compounds are considered as one of the most important groups of endocrine-disrupting chemicals (EDC) 5, 6. The presence of phthalates in the human body can lead to some diseases and harmful effects on health, such as disrupting hormone levels, infertility, increased abortion rates, hyperglycemia, cardiometabolic risk factors, kidney diseases, hypertension, thyroid gland dysfunction, and obesity. It also causes problems in the environment and aquatic organisms, including disturbances in photosynthesis, thinning, and brittleness of fish eggshells 7-9. Therefore, several techniques are developed to remove PAEs from aqueous mediums, including photocatalytic process 10, peroxi-coagulation 11, electrochemical techniques 12, 13, microbial degradation 14, 15, and adsorption 16, 17. The adsorption process offers satisfactory treatment efficiency and seems to be a more attractive approach in terms of simplicity of technique, cost-effectiveness, and applicability. Nanomaterials as adsorbents with a high specific surface area are received significant attention in the removal of many pollutants from water and wastewater. Layered double hydroxides (LDHs) are considered as one of the most widely used adsorbents for the treatment of hazardous and toxic pollutants from aqueous solutions because of their structural properties, suitable interlayer space, extraordinary ion exchange capacity, and significant removal efficiency. In the last decade, the application of LDHs for efficient adsorption of various environmental pollutants such as dyes, heavy metals, pharmaceutical compounds, petroleum compounds, fluoride, and etc. has been investigated 18-20.
The structure of LDHs can be explained by the formula, where M2+ and M3+ denote divalent cations (Ca2+, Mg2+, Ni2+, Mn2+, Zn2+, etc.) and trivalent cations (Fe3+, Co3+, Ga3+, etc.), An- denote the interlayer anions (NO3-, SO42-, Cl-, etc.) and x is defined as the ratio of M3+/(M2++M3+) 21, 22.
formula, where M2+ and M3+ denote divalent cations (Ca2+, Mg2+, Ni2+, Mn2+, Zn2+, etc.) and trivalent cations (Fe3+, Co3+, Ga3+, etc.), An- denote the interlayer anions (NO3-, SO42-, Cl-, etc.) and x is defined as the ratio of M3+/(M2++M3+) 21, 22.
In the current work, Mg-Al-LDH was synthesized and applied as the adsorbent for diethyl phthalate (DEP) removal from polluted water. The effect of initial DEP concentration, amount of Mg-Al-LDH as adsorbent, and reaction time were studied. Adsorption isotherm and kinetic studies were carried out to explain the characteristics and mechanism of the process.
Materials and Methods
Chemical
Diethyl phthalate (C6H4-1,2-(CO2C2H5)2, 99.5%, CAS number: 84-66-2) was obtained from Sigma–Aldrich. Aluminum chloride (AlCl3, 99.0%), hydrochloric acid (HCl, 37.0%), magnesium chloride (MgCl2.6H2O, 99.0%), sodium carbonate (NaCO3, ˃ 99.0%), and sodium hydroxide (NaOH, 98.0%) were purchased from Merck. All experiments were performed at 20 ± 2°C and experimental samples were prepared in double-distilled water (DDW).
Preparation of Mg-Al-LDH
The Mg-Al-LDH was prepared as the adsorbent in the DEP removal process via the co-precipitation technique. Solution (I) containing magnesium nitrate hexahydrate and aluminum nitrate nonahydrate with the molar ratio 2 to 1 of Mg2+/Al3+ was prepared in 0.15 L of DDW. Solution (II) including 10.0 g of sodium hydroxide and 26.6 g of sodium carbonate dissolved in 0.15 L DDW. Solution (I) and (II) were mixed in the reactor using a magnet stirrer, and the pH of the mixture adjusted in the alkaline range of 9-10. The composition obtained from the previous step was kept at ambient temperature (21 ± 2◦C) for 24 hours and then washed with DDW to neutralize the pH, and eventually centrifuged. Subsequently, the resulting mixture dried at 80 ° C for 2 hours in an oven. The dried product was ground and passed through a 100 mesh sieve and used as an adsorbent to remove DEP from polluted water 23.
Analysis Parametric
All experiments were performed in a pyrex reactor containing 0.2 L sample (with specified and determined conditions) at room temperature (21 ± 2◦C). The pH was measured by a pH meter (metrohm 827 pH/mV lab) and adjusted using hydrochloric acid and sodium hydroxide. After the desired reaction time, the content of the reactor was centrifuged at 1000 rpm, and the supernatant was measured to determine the amount of residual DEF. The removal efficiency of DEP and adsorption capacity were calculated by the equation (1) and (2) as follow 24, 25:
Where M0 and Me (mg L–1) denote DEP concentration before and after the adsorption process, respectively. W denotes the adsorbent dosage (g).
Adsorption kinetics and isotherm
To remove environmental pollutants in the most absorption studies, the first and second-order kinetics models are investigated. The first and second-order kinetics models expressed by Eq. 3 and Eq. 4, respectively, as follows 26, 27:

The isotherms describe the adsorption process and provide important data for designing the system. The equilibrium adsorption of DEP onto the employed Mg-Al-LDH was modeled using isotherm models of Freundlich and Langmuir by fitting the obtained results from the experiments onto the mentioned models as expressed by Eq. 5 and Eq. 6, respectively 27-29.

The value of RL as a parameter revealed that the absorption of DEP onto the adsorbent was favorable or unfavorable calculated using the following equation.

Ethical issues
The current work was conducted in the autumn of 2019, after receiving approval from the ethics committee of Kerman University of Medical Sciences [IR.KMU.REC.1398.414].
Results
Characterization of Mg-Al-LDH
The surface morphology of Mg-Al-LDH as adsorbent is showed in figure 1. The figure1 displays the characteristic of a flaky, sheeted, smooth, and relatively homogeneous surface structure similar to the reports of Wan et al. 30. Moreover, the prepared Mg-Al-LDH size was within the nanometer dimensions.
Phthalates or phthalic acid esters (PAEs) are employed as common additives in paints and resins, printing inks, lubricants, floor coverings, adhesives, insecticides, packaging, cosmetic products, and as a solvent in the rubber and plastic industries. Some types of phthalate esters are already blacklisted as priority water pollutants by the Environmental Protection Agency (EPA) 1, 2. Phthalates are not chemically bound to plastics. Therefore, they exist as freely mobile and leachable compounds. They are released from soft plastics into the environment. Phthalates have been detected in various environments such as atmosphere, surface water, sediment, and sewage sludge at various concentrations in the range of ng L-1 to mg L-1 3, 4. The presence of phthalates and their derivatives in the environment causes many concerns about human and animal health. After these compounds enter into the human and animal body, they can accumulate and concentrate in their unchanged form or metabolized form, which are detected in the intracellular or extracellular body fluids such as blood, urine, milk, etc. Since phthalates have the potential to disrupt proper endocrine function, these compounds are considered as one of the most important groups of endocrine-disrupting chemicals (EDC) 5, 6. The presence of phthalates in the human body can lead to some diseases and harmful effects on health, such as disrupting hormone levels, infertility, increased abortion rates, hyperglycemia, cardiometabolic risk factors, kidney diseases, hypertension, thyroid gland dysfunction, and obesity. It also causes problems in the environment and aquatic organisms, including disturbances in photosynthesis, thinning, and brittleness of fish eggshells 7-9. Therefore, several techniques are developed to remove PAEs from aqueous mediums, including photocatalytic process 10, peroxi-coagulation 11, electrochemical techniques 12, 13, microbial degradation 14, 15, and adsorption 16, 17. The adsorption process offers satisfactory treatment efficiency and seems to be a more attractive approach in terms of simplicity of technique, cost-effectiveness, and applicability. Nanomaterials as adsorbents with a high specific surface area are received significant attention in the removal of many pollutants from water and wastewater. Layered double hydroxides (LDHs) are considered as one of the most widely used adsorbents for the treatment of hazardous and toxic pollutants from aqueous solutions because of their structural properties, suitable interlayer space, extraordinary ion exchange capacity, and significant removal efficiency. In the last decade, the application of LDHs for efficient adsorption of various environmental pollutants such as dyes, heavy metals, pharmaceutical compounds, petroleum compounds, fluoride, and etc. has been investigated 18-20.
The structure of LDHs can be explained by the
 formula, where M2+ and M3+ denote divalent cations (Ca2+, Mg2+, Ni2+, Mn2+, Zn2+, etc.) and trivalent cations (Fe3+, Co3+, Ga3+, etc.), An- denote the interlayer anions (NO3-, SO42-, Cl-, etc.) and x is defined as the ratio of M3+/(M2++M3+) 21, 22.
formula, where M2+ and M3+ denote divalent cations (Ca2+, Mg2+, Ni2+, Mn2+, Zn2+, etc.) and trivalent cations (Fe3+, Co3+, Ga3+, etc.), An- denote the interlayer anions (NO3-, SO42-, Cl-, etc.) and x is defined as the ratio of M3+/(M2++M3+) 21, 22. In the current work, Mg-Al-LDH was synthesized and applied as the adsorbent for diethyl phthalate (DEP) removal from polluted water. The effect of initial DEP concentration, amount of Mg-Al-LDH as adsorbent, and reaction time were studied. Adsorption isotherm and kinetic studies were carried out to explain the characteristics and mechanism of the process.
Materials and Methods
Chemical
Diethyl phthalate (C6H4-1,2-(CO2C2H5)2, 99.5%, CAS number: 84-66-2) was obtained from Sigma–Aldrich. Aluminum chloride (AlCl3, 99.0%), hydrochloric acid (HCl, 37.0%), magnesium chloride (MgCl2.6H2O, 99.0%), sodium carbonate (NaCO3, ˃ 99.0%), and sodium hydroxide (NaOH, 98.0%) were purchased from Merck. All experiments were performed at 20 ± 2°C and experimental samples were prepared in double-distilled water (DDW).
Preparation of Mg-Al-LDH
The Mg-Al-LDH was prepared as the adsorbent in the DEP removal process via the co-precipitation technique. Solution (I) containing magnesium nitrate hexahydrate and aluminum nitrate nonahydrate with the molar ratio 2 to 1 of Mg2+/Al3+ was prepared in 0.15 L of DDW. Solution (II) including 10.0 g of sodium hydroxide and 26.6 g of sodium carbonate dissolved in 0.15 L DDW. Solution (I) and (II) were mixed in the reactor using a magnet stirrer, and the pH of the mixture adjusted in the alkaline range of 9-10. The composition obtained from the previous step was kept at ambient temperature (21 ± 2◦C) for 24 hours and then washed with DDW to neutralize the pH, and eventually centrifuged. Subsequently, the resulting mixture dried at 80 ° C for 2 hours in an oven. The dried product was ground and passed through a 100 mesh sieve and used as an adsorbent to remove DEP from polluted water 23.
Analysis Parametric
All experiments were performed in a pyrex reactor containing 0.2 L sample (with specified and determined conditions) at room temperature (21 ± 2◦C). The pH was measured by a pH meter (metrohm 827 pH/mV lab) and adjusted using hydrochloric acid and sodium hydroxide. After the desired reaction time, the content of the reactor was centrifuged at 1000 rpm, and the supernatant was measured to determine the amount of residual DEF. The removal efficiency of DEP and adsorption capacity were calculated by the equation (1) and (2) as follow 24, 25:
Where M0 and Me (mg L–1) denote DEP concentration before and after the adsorption process, respectively. W denotes the adsorbent dosage (g).
Adsorption kinetics and isotherm
To remove environmental pollutants in the most absorption studies, the first and second-order kinetics models are investigated. The first and second-order kinetics models expressed by Eq. 3 and Eq. 4, respectively, as follows 26, 27:

The isotherms describe the adsorption process and provide important data for designing the system. The equilibrium adsorption of DEP onto the employed Mg-Al-LDH was modeled using isotherm models of Freundlich and Langmuir by fitting the obtained results from the experiments onto the mentioned models as expressed by Eq. 5 and Eq. 6, respectively 27-29.

The value of RL as a parameter revealed that the absorption of DEP onto the adsorbent was favorable or unfavorable calculated using the following equation.

Ethical issues
The current work was conducted in the autumn of 2019, after receiving approval from the ethics committee of Kerman University of Medical Sciences [IR.KMU.REC.1398.414].
Results
Characterization of Mg-Al-LDH
The surface morphology of Mg-Al-LDH as adsorbent is showed in figure 1. The figure1 displays the characteristic of a flaky, sheeted, smooth, and relatively homogeneous surface structure similar to the reports of Wan et al. 30. Moreover, the prepared Mg-Al-LDH size was within the nanometer dimensions.
.PNG)
Figure 1: FE-SEM image of Mg–Al-LDH
As shown in Figure 2, the XRD pattern of Mg-Al-LDH revealed that the peaks of the 11.2, 24.3, 34.7, 39.1, 46.5, 61.3, 62.9, and 66.4 patterns which depending on the Mg-Al-LDH. The presence peaks pattern of 24.3, 34.7, 39.1 confirmed the hexagonal lattice of the Mg-Al-LDH 31.
.PNG)
Figure 2: The XRD pattern of Mg-Al-LDH
The graph of the isotherms obtained for Mg-Al-LDH is similar to type IV isotherms (see Figure 3), indicating that the pore structure of the Mg-Al-LDH is mainly composed of well-developed micropores. Figure 3 showed hysteresis loops at P/P0> 0.50, demonstrating the presence of certain mesoporosity in the Mg-Al-LDH. The surface area of 673 (m2 g-1), total pore of 0.716 (cm3 g-1), and microspore volumes of 0.627 (cm3 g-1), and pore diameter of 8.64 nm.

Figure 3: N2 adsorption-desorption isotherms of Mg-Al-LDH.
Investigation of initial DEP concentration
The removal efficiency of 73.2% was obtained for DEP concentration of 5 mg L-1. The percentage of the DEP removal efficiency decreased to 70.6%, 53.3%, 41.7%, and 24.1% when the concentration of the DEP was increased to 10 mg L-1, 20 mg L-1, 30 mg L-1, and 50 mg L-1, respectively (see Figure 4). It showed that the removal of the DEP was highly concentration-dependent. After applying the treatment process for 30 min, the changes in the efficiency were negligible, and the obtained curve seems smooth, and a plateau region was reached.
Investigation of the Mg-Al-LDH dosage
The effect of Mg-Al-LDH dosage on the removal efficiency of DEP was investigated in the range of 0.05 to 0.5 g L-1. The obtained results are presented in Figure 5.
The removal efficiency of 73.2% was obtained for DEP concentration of 5 mg L-1. The percentage of the DEP removal efficiency decreased to 70.6%, 53.3%, 41.7%, and 24.1% when the concentration of the DEP was increased to 10 mg L-1, 20 mg L-1, 30 mg L-1, and 50 mg L-1, respectively (see Figure 4). It showed that the removal of the DEP was highly concentration-dependent. After applying the treatment process for 30 min, the changes in the efficiency were negligible, and the obtained curve seems smooth, and a plateau region was reached.
Investigation of the Mg-Al-LDH dosage
The effect of Mg-Al-LDH dosage on the removal efficiency of DEP was investigated in the range of 0.05 to 0.5 g L-1. The obtained results are presented in Figure 5.

Figure 4: Effect of different DEP concentrations on the removal efficiency (LDH dosage of 0.15 g L-1, pH=7.5).

Figure 5: Effect of different adsorbent dosages (Mg–Al-LDH) on the removal efficiency
(DEP concentration 10 mg L-1, pH = 7.5).
As shown in the figure 5, the removal of DEP 42.1%, 51.8%, 53.3%, 75.1%, 96.7% and 97.2% were achieved at the Mg-Al-LDH dosage of 0.05, 0.10, 0.15, 0.30, 0.50, and 0.70 g L-1, respectively. The adsorbent dosage of 0.50 and 0.70 g L-1 revealed very close removal efficiencies, so the optimal adsorbent dosage of 0.50 g L-1 was considered for further studies.
Kinetics and isotherm studies
The calculated kinetics and isotherm parameters for the DEP adsorption onto the Mg-Al-LDH were summarized in Table 1 and 2, respectively.
Kinetics and isotherm studies
The calculated kinetics and isotherm parameters for the DEP adsorption onto the Mg-Al-LDH were summarized in Table 1 and 2, respectively.
Table 1: Parameters of first and second-order kinetics models for the adsorption of DEP using Mg-Al-LDH.
| First-order model | Second-order model | ||||
| qe(mg g-1) | k1(min-1) | R2 | qe(mg g-1) | k2 (g mg-1 min-1) | R2 |
| 69.41 | 0.0614 | 0.9137 | 73.4 | 0.0078 | 0.9967 |
Table 2: Langmuir and Freundlich parameters for the adsorption of DEP using Mg-Al-LDH.
| Langmuir | Freundlich | |||||
| qm (mg g-1) | b (L mg-1) | RL | R2 | Kf (L g-1) | n | R2 |
| 101.6 | 0.564 | 0.024 | 0.9907 | 45.6 | 2.18 | 0.9438 |
Discussion
According to the obtained results, the removal efficiency is greatly dependent on the DEP concentration. The effect of the pollutant concentration on the adsorption process efficiency depends on the relationship between its concentration and the accessible binding active sites of the adsorbent 27, 28. The DEP removal of the process was decreased by increasing the concentration of DEP, which may be attributed to the saturation of activated adsorption sites on the surface of adsorbent (Mg-Al-LDH). At the low concentration of DEP, there are unoccupied active sites on the surface of adsorbent (Mg-Al-LDH), whereby increasing the DEP concentration, the available active sites were saturated, which resulted in decreasing the removal efficiency of the treatment process 8, 21.
In addition, increasing the DEP concentration caused to improve in the adsorption capacity (qm), which may be because of the high driving force for mass transfer at high DEP concentrations 19, 20. Moreover, increasing Mg-Al-LDH dosage increases the DEP removal efficiency during the adsorption process, which is attributed to the more available pore volume and active surface area in higher adsorbent dosage 32, 33.
The ascending trend of the treatment process is favorable up to a reaction time of 30 min. However, by reaching the equilibrium time, the desorption of DEP has occurred, and the removal rate was decreased negligibly. Therefore, the equilibrium time was set to 30 min. The obtained experimental data showed that the second-order kinetics model revealed a better agreement with the higher correlation coefficient (R2 = 0.99) than the first-order kinetics model (R2 = 0.91). The second-order kinetic model confirmed that the rate-limiting step of the treatment process in the current work is controlled under the chemisorption mechanism 22-25. The obtained isotherm results better fitted to the Langmuir model with a higher correlation coefficient
(R2 = 0.99) compared to the Freundlich model (R2 = 0.94). The maximum DEP adsorption capacity (qm) was found to be 101.6 mg g-1. The obtained values of n and RL were 2.18 and 0.024, respectively.
Conclusion
DEP and its derivatives in the environment cause many concerns about human and animal health and adverse environmental effects. The current work was focused on the efficiency of adsorbent (Mg-Al-LDH) in DEP removal from polluted water. In the optimum condition, including DEP concentration of 10 mg L-1, Mg-Al-LDH dosage of 0.50 g L-1, and the reaction time of 30 min, the maximum removal efficiency and maximum adsorption capacity were found to be 96.7% and 101.6 mg g-1. The experimental results showed that the Mg-Al-LDH could be successfully employed as an effective and economical adsorbent to remove hazardous pollutants such as DEP from polluted water.
Acknowledgments
The authors would like to express their appreciation to the student research committee of Kerman University of Medical Sciences for supporting the current work.
Funding
This work received a grant from the Kerman University of Medical Sciences [Grant No. 98000185].
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of the current paper.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
According to the obtained results, the removal efficiency is greatly dependent on the DEP concentration. The effect of the pollutant concentration on the adsorption process efficiency depends on the relationship between its concentration and the accessible binding active sites of the adsorbent 27, 28. The DEP removal of the process was decreased by increasing the concentration of DEP, which may be attributed to the saturation of activated adsorption sites on the surface of adsorbent (Mg-Al-LDH). At the low concentration of DEP, there are unoccupied active sites on the surface of adsorbent (Mg-Al-LDH), whereby increasing the DEP concentration, the available active sites were saturated, which resulted in decreasing the removal efficiency of the treatment process 8, 21.
In addition, increasing the DEP concentration caused to improve in the adsorption capacity (qm), which may be because of the high driving force for mass transfer at high DEP concentrations 19, 20. Moreover, increasing Mg-Al-LDH dosage increases the DEP removal efficiency during the adsorption process, which is attributed to the more available pore volume and active surface area in higher adsorbent dosage 32, 33.
The ascending trend of the treatment process is favorable up to a reaction time of 30 min. However, by reaching the equilibrium time, the desorption of DEP has occurred, and the removal rate was decreased negligibly. Therefore, the equilibrium time was set to 30 min. The obtained experimental data showed that the second-order kinetics model revealed a better agreement with the higher correlation coefficient (R2 = 0.99) than the first-order kinetics model (R2 = 0.91). The second-order kinetic model confirmed that the rate-limiting step of the treatment process in the current work is controlled under the chemisorption mechanism 22-25. The obtained isotherm results better fitted to the Langmuir model with a higher correlation coefficient
(R2 = 0.99) compared to the Freundlich model (R2 = 0.94). The maximum DEP adsorption capacity (qm) was found to be 101.6 mg g-1. The obtained values of n and RL were 2.18 and 0.024, respectively.
Conclusion
DEP and its derivatives in the environment cause many concerns about human and animal health and adverse environmental effects. The current work was focused on the efficiency of adsorbent (Mg-Al-LDH) in DEP removal from polluted water. In the optimum condition, including DEP concentration of 10 mg L-1, Mg-Al-LDH dosage of 0.50 g L-1, and the reaction time of 30 min, the maximum removal efficiency and maximum adsorption capacity were found to be 96.7% and 101.6 mg g-1. The experimental results showed that the Mg-Al-LDH could be successfully employed as an effective and economical adsorbent to remove hazardous pollutants such as DEP from polluted water.
Acknowledgments
The authors would like to express their appreciation to the student research committee of Kerman University of Medical Sciences for supporting the current work.
Funding
This work received a grant from the Kerman University of Medical Sciences [Grant No. 98000185].
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of the current paper.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
- Net S, Delmont A, Sempéré R, et al. Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Sci Total Environ. 2015;515:162-80.
- Gao D-W, Wen Z-D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ. 2016;541:986-1001.
- Dargnat C, Teil M-J, Chevreuil M, et al. Phthalate removal throughout wastewater treatment plant: case study of Marne Aval station (France). Sci Total Environ. 2009;407(4):1235-44.
- Pang X, Skillen N, Gunaratne N, et al. Removal of phthalates from aqueous solution by semiconductor photocatalysis: A review. J Hazard Mater. 2020;402:123461.
- Khosravi K, Price GW. Determination of phthalates in soils and biosolids using accelerated solvent extraction coupled with SPE cleanup and GC–MS quantification. Microchem J. 2015;121:205-12.
- Deblonde T, Cossu-Leguille C, Hartemann P. Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health. 2011;214(6):442-8.
- Kotowska U, Kapelewska J, Sawczuk R. Occurrence, removal, and environmental risk of phthalates in wastewaters, landfill leachates, and groundwater in Poland. Environ Pollut. 2020;267:115643.
- Dolatabadi M, Ahmadzadeh S, Ghaneian MT. Mineralization of mefenamic acid from hospital wastewater using electro‐Fenton degradation: Optimization and identification of removal mechanism issues. Environ Prog Sustain Energy. 2020;39(3): 13380.
- Rivera-Utrilla J, Ocampo-Pérez R, Méndez-Díaz JD, et al. Environmental impact of phthalic acid esters and their removal from water and sediments by different technologies–a review. J Environ Manag. 2012;109:164-78.
- Gu X, Qin N, Wei G, et al. Efficient photocatalytic removal of phthalates easily implemented over a bi-functional TiO2 surface. Chemosphere. 2021;263:128257.
- Ding J, Dong L, Geng Y, et al. Modification of graphite felt doped with nitrogen and boron for enhanced removal of dimethyl phthalate
in peroxi-coagulation system and mechanisms. Environ Sci Pollut Res. 2020;27(15): 18810-21. - Zaied B, Rashid M, Nasrullah M, et al. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci Total Environ. 2020;726:138095.
- GilPavas E, Dobrosz-Gómez I, Gómez-García M-Á. Efficient treatment for textile wastewater through sequential electrocoagulation, electrochemical oxidation and adsorption processes: Optimization and toxicity assessment. J Electroanal Chem. 2020;878:114578.
- Boll M, Geiger R, Junghare M, et al. Microbial degradation of phthalates: biochemistry and environmental implications. Environ Microbiol Rep. 2020;12(1):3-15.
- Chen C-Y, Wu P-S, Chung Y-C. Coupled biological and photo-Fenton pretreatment system for the removal of di-(2-ethylhexyl) phthalate (DEHP) from water. Bioresour Technol. 2009;100(19):4531-4.
- Prasanna VL, Mamane H, Vadivel VK, et al. Ethanol-activated granular aerogel as efficient adsorbent for persistent organic pollutants from real leachate and hospital wastewater. J Hazard Mater. 2020;384:121396.
- Eslami A, Saghi MH, Akbari-adergani B, et al. Synthesis of modified ZnO nanorods and investigation of its application for removal of phthalate from landfill leachate: A case study in Aradkouh landfill site. J Environ Health Sci Eng. 2021:1-10.
- Theiss FL, Couperthwaite SJ, Ayoko GA, et al. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J Colloid Interface Sci. 2014;417:356-68.
- Wen T, Wu X, Tan X, et al. One-pot synthesis of water-swellable Mg–Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As (V) from aqueous solutions. ACS Appl Mater Interfaces. 2013;5(8):3304-11.
- Goh KH, Lim TT, Dong Z. Application of layered double hydroxides for removal of oxyanions: a review. Water Res. 2008;42(6-7):1343-68.
- Wang J, Wang X, Tan L, et al. Performances and mechanisms of Mg/Al and Ca/Al layered double hydroxides for graphene oxide removal from aqueous solution. Chem. Eng. J. 2016;297:106-15.
- Laipan M, Yu J, Zhu R, et al. Functionalized layered double hydroxides for innovative applications. Mater Horiz. 2020;7(3):715-45.
- Shan Rr, Yan Lg, Yang Ym, et al. Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J Ind Eng Chem. 2015;21:561-8.
- Jamali‐Behnam F, Najafpoor AA, Davoudi M, et al. Adsorptive removal of arsenic from aqueous solutions using magnetite nanoparticles and silica‐coated magnetite nanoparticles. Environ Prog Sustain Energy. 2018;37(3):951-60.
- Abdollahi Y, Abdullah AH, Gaya UI, et al. Photocatalytic degradation of 1, 4-benzoquinone in aqueous ZnO dispersions. J Braz Chem Soc. 2012;23(2):236-40.
- Rezayi M, Heng LY, Kassim A, et al. Immobilization of tris (2 pyridyl) methylamine in a PVC-Membrane Sensor and Characterization of the Membrane Properties. Chem. Cent. J. 2012;6(1):1-6.
- Najafpoor A, Alidadi H, Esmaeili H, et al. Optimization of anionic dye adsorption onto Melia azedarach sawdust in aqueous solutions: effect of calcium cations. Asia-Pac J Chem Eng. 2016;11(2):258-70.
- Ali I, Alharbi OM, ALOthman ZA, et al. Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ Res. 2019;170:389-97.
- Sabarinathan C, Karuppasamy P, Vijayakumar C, et al. Development of methylene blue removal methodology by adsorption using molecular polyoxometalate: Kinetics, Thermodynamics and Mechanistic Study. Microchem J. 2019;146:315-26.
- Wan S, Wang S, Li Y, et al. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J Ind Eng Chem. 2017;47:246-53.
- Lyu F, Yu H, Hou T, et al. Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J Colloid Interface Sci. 2019;539:184-93.
- Tong Y, McNamara PJ, Mayer BK. Adsorption of organic micropollutants onto biochar: a review of relevant kinetics, mechanisms and equilibrium. Environ Sci Water Res Technol. 2019;5(5):821-38.
- Garba ZN, Zhou W, Lawan I, et al. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J Environ Manage. 2019;241:59-75.
Type of Study: Original articles |
Subject:
Environmental pollution
Received: 2021/05/11 | Accepted: 2021/07/20 | Published: 2021/09/25
Received: 2021/05/11 | Accepted: 2021/07/20 | Published: 2021/09/25
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








