Volume 3, Issue 1 (March 2018)
J Environ Health Sustain Dev 2018, 3(1): 481-487 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khoshnamvand N, Bazrafshan E, Kamarei B. Fluoride Removal from Aqueous Solutions by NaOH-Modified Eucalyptus Leaves
. J Environ Health Sustain Dev 2018; 3 (1) :481-487
URL: http://jehsd.ssu.ac.ir/article-1-103-en.html
URL: http://jehsd.ssu.ac.ir/article-1-103-en.html
Nutritional Health Research Center , Lorestan University of Medical Sciences, Khorramabad, Iran.
Full-Text [PDF 684 kb]
(890 Downloads)
| Abstract (HTML) (3493 Views)
Citation: Khoshnamvand N, Bazrafshan E, Kamarei B. Fluoride Removal from Aqueous Solutions by NaOH-Modified Eucalyptus Leaves. J Environ Health Sustain Dev. 2018; 3(1):481-7.

Figure 1: The effect of pH on fluoride adsorption in modified eucalyptus leaves
(Initial F concentration 20 mg/L, adsorbent dosage = 0.5 g/L, time = 60 min)
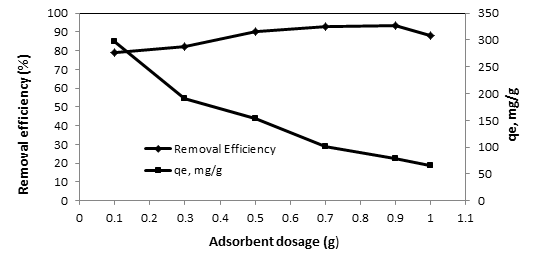
Figure 2: The effect of adsorbent dosage on fluoride adsorption by modified eucalyptus leaves
(Initial F concentration = 20 mg/L, initial pH = 2, time = 60 min)

Figure 3: The effect of fluoride concentration on contaminant adsorption by modified eucalyptus leaves
(Optimum pH = 2, time = 60 min, adsorbent dosage = 0.5 g/L)


Figure 4: The effect of the Contact Time on fluoride adsorption by modified eucalyptus leaves
(Optimum pHm = 2, adsorbent dosage = 0.5 g/L)
Table 1: Kinetic parameters used in models
Full-Text: (1617 Views)
Fluoride Removal from Aqueous Solutions by NaOH-Modified Eucalyptus Leaves
Nahid Khoshnamvand 1*, Edris Bazrafshan 2, Bahram Kamarei 3
1 Nutritional Health Research Center , Lorestan University of Medical Sciences, Khorramabad, Iran.
2 Department of Environmental Health, Torbat Heydarieh University of Medical Sciences, Torbat Heydarieh, Iran.
3 Nutritional Health Research Center , Lorestan University of Medical Sciences, Khorramabad, Iran.
Nahid Khoshnamvand 1*, Edris Bazrafshan 2, Bahram Kamarei 3
1 Nutritional Health Research Center , Lorestan University of Medical Sciences, Khorramabad, Iran.
2 Department of Environmental Health, Torbat Heydarieh University of Medical Sciences, Torbat Heydarieh, Iran.
3 Nutritional Health Research Center , Lorestan University of Medical Sciences, Khorramabad, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Fluoride (F) and its compounds are widely used in industries in which fluoride overdose leads to various detrimental diseases. In this study the effect of NaOH-modified Eucalyptus leaves on fluoride removal from aqueous solutions as a natural adsorbent was investigated. Materials and Methods: The focus of this study was on the effects of parameters such as pH (2-12), initial concentration (5-30 mg/L), adsorbent dose (0.1-1 g/L) and temperature (25-45 0C). Fluoride residual was measured using the SPADNS method by a DR 5000 spectrophotometer. Results: The maximum adsorption capacity, at pH 2 was found to be 97 mg F/g with an adsorbent dosage of 0.5 g/L and an initial fluoride concentration of 20 mg/L. The adsorption equilibrium and kinetic data were in good agreement with Langmuir Model (R2 = 0.967) with qmax = 298 mg/g and pseudo-second order reaction (R2 = 0.999). Conclusion: Based on the results, NaOH-modified eucalyptus leaves were found to be able to remove fluoride from aqueous environments with good removal efficiency and adsorption capacity. |
|
| Article History: Received: 31 October 2017 Accepted: 20 January 2018 |
||
| *Corresponding Author: Nahid khoshnamvand Email: Nahidkhoshnam92@gmail.com Tel: +989169692080 |
||
| Keywords: Eucalyptus, Fluoride Removal, Natural Adsorbent. |
Citation: Khoshnamvand N, Bazrafshan E, Kamarei B. Fluoride Removal from Aqueous Solutions by NaOH-Modified Eucalyptus Leaves. J Environ Health Sustain Dev. 2018; 3(1):481-7.
Introduction
Fluoride (F) has both beneficial and harmful effects on human health depending on its level 1. Depending on the concentration and duration of continuous absorption, the absorption of high amounts of fluoride could lead to osteoporosis, arthritis, brittle bones, cancer, infertility, brain damage, Alzheimer's disease, stained teeth, impaired DNA synthesis, and impaired metabolism of carbohydrates, lipids, proteins, vitamins and minerals 2. The World Health Organization (WHO) has specified F tolerance limit in drinking water as 1.5 mg/L 3. The ion-fluoride is the most electronegative ion, which tends to be combined with various compounds such as potassium, aluminum and zinc 4. Fluoride is in different environments such as air, underground water, drinking water, bottled water and some forms of black tea 5. In areas rich in minerals containing fluoride, its concentration in groundwater is found to be 10 mg/l. The maximum F concentration of in water has been reported at 2800 mg/l in the world 3. Various techniques have been proposed for F removal from aqueous environments including adsorption, membrane separation, ion-exchange, precipitation, coagulation, nano-filtration and electrolytic defluoridation 6. Furthermore, there are several types of coagulants such as alum, ferric sulfate, ferro sulfate, ferric chloride, organic anionic, cationic and nonionic polymers 7- 9. For the removal of organic and mineral contaminants the method of adsorption is a cheap and cost-effective, which are performed by: chitosan beads and carbon-based materials. Eucalyptus (eucalyptus camadulensis Dehnh) is abundantly found in the south-western and south-eastern parts of Iran. The aim of this study was to evaluate the efficiency of NaOH-modified Eucalyptus leaves in F removal from water.
Materials and Methods
 Firstly, Eucalyptus leaves were collected from trees in Zahedan city, and washed with distilled water and dried at 110 0C for 2 hr. Then the dried leaves were powdered into 60 to 200 mesh sizes using standard ASTM sieves. To modify the adsorbent, 250 grams of the powder were then mixed with NaOH and left at normal room temperature for 3 hr. Preparation and modification of adsorbent was initially done based on the method presented by Khavidaki et al. and Fazlzadeh et al., respectively with some changes 10, 11 .A stock F solution was prepared by dissolving 0.221 g of NaF in 1000 mL of distilled water. For each experimental run, 100 mL of F solution of known concentration (5–30 mg/L), pH (2–12), and a known amount of the adsorbent (0.1–1.0 g/L) were taken in a 100 mL stoppered conical flask. Then, 100 mL of F solution was mixed with various doses of the adsorbent at different pH and F concentrations at equilibrium time in a reciprocating shaker at 200 rpm after determining the optimal points. After that, an investigation of the effects of various temperatures (25-45 0C) on F concentrations was conducted. In order to adjust pH, normal NaOH and H2SO4 solutions were
Firstly, Eucalyptus leaves were collected from trees in Zahedan city, and washed with distilled water and dried at 110 0C for 2 hr. Then the dried leaves were powdered into 60 to 200 mesh sizes using standard ASTM sieves. To modify the adsorbent, 250 grams of the powder were then mixed with NaOH and left at normal room temperature for 3 hr. Preparation and modification of adsorbent was initially done based on the method presented by Khavidaki et al. and Fazlzadeh et al., respectively with some changes 10, 11 .A stock F solution was prepared by dissolving 0.221 g of NaF in 1000 mL of distilled water. For each experimental run, 100 mL of F solution of known concentration (5–30 mg/L), pH (2–12), and a known amount of the adsorbent (0.1–1.0 g/L) were taken in a 100 mL stoppered conical flask. Then, 100 mL of F solution was mixed with various doses of the adsorbent at different pH and F concentrations at equilibrium time in a reciprocating shaker at 200 rpm after determining the optimal points. After that, an investigation of the effects of various temperatures (25-45 0C) on F concentrations was conducted. In order to adjust pH, normal NaOH and H2SO4 solutions were
used. Afterwards, the solutions were filtered (0.45 μ m, Whatman filter paper), and then the residual F concentration was analyzed at a maximum wavelength of 570 nm using UV/VIS spectrophotometer (PerkinElmer, Lambda 25) according to the standard methods for water and wastewater examination 12.
In order to determine the pH of zero point of charge (pHzpc), 0.01 M NaCl was prepared and the suspension pH was adjusted within the range of 2.0–12.0 using 0.1 M NaOH and HCl. Then 50 mL different pH of 0.01 M NaCl solution was taken into a conical flask containing 0.5 g adsorbents. The mixtures were shaken for 24 hr and then the final pH was measured 13 The amount of adsorbed fluoride at equilibrium (qe) (mg/g) was calculated using equation 1, and F removal efficiency (%) was calculated using equation 2:
.png) (equation 1)
(equation 1)
.png) (equation 2)
(equation 2)
Where,
qe: Adsorption capacity (mg/g)
C0: Initial concentration of fluoride in the solution (mg/l)
Ce: Equilibrium concentration of fluoride after the establishment of equilibrium (mg/l)
V: Solution volume (L)
M: Adsorbent mass (g)
Results
To achieve the studies goal, after preparing and modifying the adsorbent, the adsorption equilibrium time was determined, and then the effects of various variables on the adsorption efficiency and capacity were studied by conducting batch experiments.
In this paper, the effect of pH (2–12) on F removal in aqueous solution was studied. As shown in Figure 1. The adsorbent dosage is an important factor, since it determines the capacity of the adsorbent for a given initial F concentration as shown in Figure 2. The results of F removal efficiency at the optimal pH and adsorbent dosage with various initial concentrations and contact time of F are presented in Figure 3 and 4. Adsorption amounts at various times were investigated using pseudo first-order and pseudo second-order kinetic models. The results of the matching are presented in Table 1.
Fluoride (F) has both beneficial and harmful effects on human health depending on its level 1. Depending on the concentration and duration of continuous absorption, the absorption of high amounts of fluoride could lead to osteoporosis, arthritis, brittle bones, cancer, infertility, brain damage, Alzheimer's disease, stained teeth, impaired DNA synthesis, and impaired metabolism of carbohydrates, lipids, proteins, vitamins and minerals 2. The World Health Organization (WHO) has specified F tolerance limit in drinking water as 1.5 mg/L 3. The ion-fluoride is the most electronegative ion, which tends to be combined with various compounds such as potassium, aluminum and zinc 4. Fluoride is in different environments such as air, underground water, drinking water, bottled water and some forms of black tea 5. In areas rich in minerals containing fluoride, its concentration in groundwater is found to be 10 mg/l. The maximum F concentration of in water has been reported at 2800 mg/l in the world 3. Various techniques have been proposed for F removal from aqueous environments including adsorption, membrane separation, ion-exchange, precipitation, coagulation, nano-filtration and electrolytic defluoridation 6. Furthermore, there are several types of coagulants such as alum, ferric sulfate, ferro sulfate, ferric chloride, organic anionic, cationic and nonionic polymers 7- 9. For the removal of organic and mineral contaminants the method of adsorption is a cheap and cost-effective, which are performed by: chitosan beads and carbon-based materials. Eucalyptus (eucalyptus camadulensis Dehnh) is abundantly found in the south-western and south-eastern parts of Iran. The aim of this study was to evaluate the efficiency of NaOH-modified Eucalyptus leaves in F removal from water.
Materials and Methods
 Firstly, Eucalyptus leaves were collected from trees in Zahedan city, and washed with distilled water and dried at 110 0C for 2 hr. Then the dried leaves were powdered into 60 to 200 mesh sizes using standard ASTM sieves. To modify the adsorbent, 250 grams of the powder were then mixed with NaOH and left at normal room temperature for 3 hr. Preparation and modification of adsorbent was initially done based on the method presented by Khavidaki et al. and Fazlzadeh et al., respectively with some changes 10, 11 .A stock F solution was prepared by dissolving 0.221 g of NaF in 1000 mL of distilled water. For each experimental run, 100 mL of F solution of known concentration (5–30 mg/L), pH (2–12), and a known amount of the adsorbent (0.1–1.0 g/L) were taken in a 100 mL stoppered conical flask. Then, 100 mL of F solution was mixed with various doses of the adsorbent at different pH and F concentrations at equilibrium time in a reciprocating shaker at 200 rpm after determining the optimal points. After that, an investigation of the effects of various temperatures (25-45 0C) on F concentrations was conducted. In order to adjust pH, normal NaOH and H2SO4 solutions were
Firstly, Eucalyptus leaves were collected from trees in Zahedan city, and washed with distilled water and dried at 110 0C for 2 hr. Then the dried leaves were powdered into 60 to 200 mesh sizes using standard ASTM sieves. To modify the adsorbent, 250 grams of the powder were then mixed with NaOH and left at normal room temperature for 3 hr. Preparation and modification of adsorbent was initially done based on the method presented by Khavidaki et al. and Fazlzadeh et al., respectively with some changes 10, 11 .A stock F solution was prepared by dissolving 0.221 g of NaF in 1000 mL of distilled water. For each experimental run, 100 mL of F solution of known concentration (5–30 mg/L), pH (2–12), and a known amount of the adsorbent (0.1–1.0 g/L) were taken in a 100 mL stoppered conical flask. Then, 100 mL of F solution was mixed with various doses of the adsorbent at different pH and F concentrations at equilibrium time in a reciprocating shaker at 200 rpm after determining the optimal points. After that, an investigation of the effects of various temperatures (25-45 0C) on F concentrations was conducted. In order to adjust pH, normal NaOH and H2SO4 solutions wereused. Afterwards, the solutions were filtered (0.45 μ m, Whatman filter paper), and then the residual F concentration was analyzed at a maximum wavelength of 570 nm using UV/VIS spectrophotometer (PerkinElmer, Lambda 25) according to the standard methods for water and wastewater examination 12.
In order to determine the pH of zero point of charge (pHzpc), 0.01 M NaCl was prepared and the suspension pH was adjusted within the range of 2.0–12.0 using 0.1 M NaOH and HCl. Then 50 mL different pH of 0.01 M NaCl solution was taken into a conical flask containing 0.5 g adsorbents. The mixtures were shaken for 24 hr and then the final pH was measured 13 The amount of adsorbed fluoride at equilibrium (qe) (mg/g) was calculated using equation 1, and F removal efficiency (%) was calculated using equation 2:
.png) (equation 1)
(equation 1).png) (equation 2)
(equation 2)Where,
qe: Adsorption capacity (mg/g)
C0: Initial concentration of fluoride in the solution (mg/l)
Ce: Equilibrium concentration of fluoride after the establishment of equilibrium (mg/l)
V: Solution volume (L)
M: Adsorbent mass (g)
Results
To achieve the studies goal, after preparing and modifying the adsorbent, the adsorption equilibrium time was determined, and then the effects of various variables on the adsorption efficiency and capacity were studied by conducting batch experiments.
In this paper, the effect of pH (2–12) on F removal in aqueous solution was studied. As shown in Figure 1. The adsorbent dosage is an important factor, since it determines the capacity of the adsorbent for a given initial F concentration as shown in Figure 2. The results of F removal efficiency at the optimal pH and adsorbent dosage with various initial concentrations and contact time of F are presented in Figure 3 and 4. Adsorption amounts at various times were investigated using pseudo first-order and pseudo second-order kinetic models. The results of the matching are presented in Table 1.

Figure 1: The effect of pH on fluoride adsorption in modified eucalyptus leaves
(Initial F concentration 20 mg/L, adsorbent dosage = 0.5 g/L, time = 60 min)

Figure 2: The effect of adsorbent dosage on fluoride adsorption by modified eucalyptus leaves
(Initial F concentration = 20 mg/L, initial pH = 2, time = 60 min)

Figure 3: The effect of fluoride concentration on contaminant adsorption by modified eucalyptus leaves
(Optimum pH = 2, time = 60 min, adsorbent dosage = 0.5 g/L)


Figure 4: The effect of the Contact Time on fluoride adsorption by modified eucalyptus leaves
(Optimum pHm = 2, adsorbent dosage = 0.5 g/L)
Table 1: Kinetic parameters used in models
| Kinetic models | Kinetic parameters | Kinetic parameter values |
| Pseudo first-order kinetic model | qe (exp) (mg/g) | 15 |
| qe (cal) (mg/g) | 2.35 | |
| K | -0.015 | |
| R2 | 0.9679 | |
| Pseudo second-order kinetic model | qe (exp) (mg/g) | 15 |
| qe (cal) (mg/g) | 15.15 | |
| K | 0.08 | |
| R2 | 0.999 |
Discussion
It has been proven that pH value plays a significant role on the system behavior in organic compounds adsorption 14. The amount of fluoride absorption depends on the pH of the solution, the amount of adsorption decreases with increasing pH. The effect of pH depends on zero point charge of catalyst and acidity constant (pKa) 15. At pH values lower than the pHzpc of eucalyptus leaves (11.2), there is a degree of attraction between positively charged eucalyptus leaves and negatively charged F. However, an optimal pH 2 was considered for investigation due to the low slope of efficiency in alkaline conditions, in comparison with acidic conditions 16- 18. Most studies have reported the highest F removal efficiency in acidic conditions. In alkaline conditions, ion OH- acts as a competitor to absorb F anions and occupy absorption sites, which results in reduced removal efficiency. By increasing the pH, the number of negative loads increases and the electrostatic gravity of the adsorbent and pollutant decreases 19. The dependence of F adsorption on the amount of NaOH-modified Eucalyptus leaves was investigated at room temperature (21 ± 20C) and at pH 2 by varying the adsorbent amount from 0.1 to 1.0 g/l in contact with 100 mL solution of 20 mg F/L As the adsorbent dosage increases from 0.1 to 1 g/L, the removal efficiency and adsorption capacity decreases. The highest F removal efficiency (93.2%) was achieved at the adsorbent dosage of 0.9 g/L at the optimal pH and the equilibrium time. However, the adsorbent dose of 0.5 g/L was used in the following stages. While the adsorption capacity (qe) decreased from about 298 to 66 mg/g with an increase in the adsorbent dose. In doses higher than 0.5, the removal efficiency was minor and choosing a higher dose was not economically feasible. The F adsorbed per gram of adsorbent decreased with an increase in the amount of adsorbent. The maximum adsorption capacity was 298 mg/g in the minimum adsorbent dose of 0.1 g/L. This is due to unsaturated active sites being present on the adsorbent surface at the certain F concentration of 30 mg/L and particle aggregation occurring with increased adsorbent concentration. At the initial stage, there are plenty of empty spaces available for absorbing the absorbent material, but increasing the amount of absorbent dose leads to absorbent levels overlapping and thus reducing the available surface area, which has a slight increase in efficiency or reduced removal of contaminants 20. This is a fact that time fit is an important factor affecting cost and energy significantly on the process. The effect of contact time on F adsorption by eucalyptus leaves was investigated for 150 min at different initial F concentrations. The removal efficiency increased with lengthy contact time and reached equilibrium at about 6 min for different initial concentrations. Therefore, 60 min was selected as the optimum contact time for other experiments. When the initial F concentration is increased, the percent of F removal decreased. In contrast, when the initial F concentration is increased, the amounts of adsorbed F also increase. The highest and lowest removal efficiencies were achieved at the initial F concentrations of 5 and 30 mg/L, respectively. As figure 3 reveals, F adsorption capacity by the utilized adsorbent increases as the initial F concentration increases. In the present study, the experimental data of the adsorption equilibrium were investigated using Langmuir and Freundlich isotherm models. The isotherm provides a relationship between the pollutant concentration in solution and the amount of pollutant adsorbed on to the solid phase when both phases are in equilibrium. According to the results, the correlation coefficient of the Langmuir model (R2 = 0.991) was higher than the Freundlich model, indicating that the Langmuir model is suitable for describing F adsorption equilibrium on NaOH -modified Eucalyptus leaves.
Investigation of Adsorption Kinetics
The obtained results indicate that F adsorption on modified eucalyptus complies with the pseudo second-order kinetic model with R2 equal to 0.999.
Conclusion
The results of this study showed that any modification of eucalyptus leaves with
NaOH could lead to surface F adsorption from aqueous solutions with maximum adsorption capacity of 298 mg/g under the optimal adsorption conditions of a pH 2. Initial concentration of 5 mg/L, and the adsorbent dosage of 0.5 g/L, gives a maximum removal efficiency of 97%. It was also documented that the pseudo-second order kinetic model and the Langmuir isotherm model had the best fit with the experimental data, and F adsorption process was facilitated. Eucalyptus modified leaves can be used under optimum conditions to remove fluoride from aqueous solutions.
Acknowledgments
Thanks go to Zahedan University of Medical Sciences for its technical and financial support of this study.
Funding
This study has been supported by the Health Enhancement Research Center of the Zahedan University of Medical Sciences (Project code: 7425).
Conflict of interests
No conflict of interest has been stated by the authors.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use.
Reference
1. Srimurali M, Pragathi A, Karthikeyan J. A study on removal of fluorides from drinking water by adsorption onto low-cost materials. Environmental Pollution. 1998; 99(2): 285-9. doi: https:// doi.org/ 10.1016/S0269-7491(97) 00129- 2.
2. Mahramanlioglu M, Kizilcikli I, Bicer I. Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem. 2002; 115(1): 41-7.
3.WHO. Guidelines for drinking-water quality 2011. 104-8 p. DOI: 10.1016/j. waters. 2007. 04. 029.
4. Mohapatra M, Rout K, Singh P, et al. Fluoride adsorption studies on mixed-phase nano iron oxides prepared by surfactant mediation-precipitation technique. Journal of hazardous materials. 2011; 186(2): 1751-7.
5. De la Puente G, Pis J, Menendez J, et al. Thermal stability of oxygenated functions in activated carbons. J Anal Appl Pyrolysis. 1997; 43(2): 125-38.
6. Ho LN, Ishihara T, Ueshima S, et al. Removal of fluoride from water through ion exchange by mesoporous Ti oxohydroxide. Journal of colloid and interface science. 2004; 272(2): 399-403.
7. Wu X, Zhang Y, Dou X, et al. Fluoride removal performance of a novel Fe–Al–Ce trimetal oxide adsorbent. Chemosphere. 2007; 69(11): 1758-64.
8. Kemer B, Ozdes D, Gundogdu A, et al. Removal of fluoride ions from aqueous solution by waste mud. Journal of hazardous materials. 2009; 168(2): 888-94.
9. Çengeloğlu Y, Kır E, Ersöz M. Removal of fluoride from aqueous solution by using red mud. Sep Purif Technol. 2002; 28(1): 81-6.
10. Fazlzadeh M, Hazrati S, Adhami S. Modification of green clay by HCl and H2SO4 to remove humic acid from aqueous solutions. Journal of Occupational and Environmental Health. 2016; 2(1): 27-46.
11. Dashti Khavidaki H, Aghaie H. Adsorption of thallium (I) ions using eucalyptus leaves powder. CLEAN–Soil, Air, Water. 2013; 41(7): 673-9.
12. Federation WE, Association AP. Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC, USA. 2005.
13. Faraji M, Bazrafshan E, Almasian M, et al. Investigation of fluoride adsorption from aqueous solutions by modified eucalyptus leaves: isotherm and kinetic and thermodynamic studies. Iranian Journal of Health Sciences. 2017; 5(3): 65-77.
14. Avisar D, Primor O, Gozlan I, et al. Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water, Air, & Soil Pollution. 2010; 209(1-4): 439-50.
15. Jonidi-Jafari A, Shirzad-Siboni M, Yang
J-K, et al. Photocatalytic degradation of diazinon with illuminated ZnO–TiO 2 composite. J Taiwan Inst Chem Eng. 2015; 50(10): 100-7.
16. Bazrafshan E, Mostafapour FK, Hosseini AR, et al. Decolorisation of reactive red 120 dye by using single-walled carbon nanotubes in aqueous solutions. Journal of Chemistry. 2013.
17. Zhang G, He Z, Xu W. A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chemical Engineering Journal. 2012; 183: 315-24.
18. Bazrafshan E, Khoshnamvand N, Mahvi AH. fluoride removal from aqueous environments by zncl 2-treated eucalyptus leaves as a natural adsorbent. Fluoride. 2015; 48(4): 196-202.
19. Zazouli MA, Belarak D, Karimnezhad F, et al. Removal of fluoride from aqueous solution by using of adsorption onto modified Lemna minor: Adsorption isotherm and kinetics study. Journal of Mazandaran University of Medical Sciences. 2014; 23(109): 195-204.
20. Samarghandy MR, Hoseinzadeh E, Taghavi M, et al. Biosorption of reactive black 5 from aqueous solution using acid-treated biomass of potato peel waste. Bio Resources. 2011; 6(4): 4840-55.
It has been proven that pH value plays a significant role on the system behavior in organic compounds adsorption 14. The amount of fluoride absorption depends on the pH of the solution, the amount of adsorption decreases with increasing pH. The effect of pH depends on zero point charge of catalyst and acidity constant (pKa) 15. At pH values lower than the pHzpc of eucalyptus leaves (11.2), there is a degree of attraction between positively charged eucalyptus leaves and negatively charged F. However, an optimal pH 2 was considered for investigation due to the low slope of efficiency in alkaline conditions, in comparison with acidic conditions 16- 18. Most studies have reported the highest F removal efficiency in acidic conditions. In alkaline conditions, ion OH- acts as a competitor to absorb F anions and occupy absorption sites, which results in reduced removal efficiency. By increasing the pH, the number of negative loads increases and the electrostatic gravity of the adsorbent and pollutant decreases 19. The dependence of F adsorption on the amount of NaOH-modified Eucalyptus leaves was investigated at room temperature (21 ± 20C) and at pH 2 by varying the adsorbent amount from 0.1 to 1.0 g/l in contact with 100 mL solution of 20 mg F/L As the adsorbent dosage increases from 0.1 to 1 g/L, the removal efficiency and adsorption capacity decreases. The highest F removal efficiency (93.2%) was achieved at the adsorbent dosage of 0.9 g/L at the optimal pH and the equilibrium time. However, the adsorbent dose of 0.5 g/L was used in the following stages. While the adsorption capacity (qe) decreased from about 298 to 66 mg/g with an increase in the adsorbent dose. In doses higher than 0.5, the removal efficiency was minor and choosing a higher dose was not economically feasible. The F adsorbed per gram of adsorbent decreased with an increase in the amount of adsorbent. The maximum adsorption capacity was 298 mg/g in the minimum adsorbent dose of 0.1 g/L. This is due to unsaturated active sites being present on the adsorbent surface at the certain F concentration of 30 mg/L and particle aggregation occurring with increased adsorbent concentration. At the initial stage, there are plenty of empty spaces available for absorbing the absorbent material, but increasing the amount of absorbent dose leads to absorbent levels overlapping and thus reducing the available surface area, which has a slight increase in efficiency or reduced removal of contaminants 20. This is a fact that time fit is an important factor affecting cost and energy significantly on the process. The effect of contact time on F adsorption by eucalyptus leaves was investigated for 150 min at different initial F concentrations. The removal efficiency increased with lengthy contact time and reached equilibrium at about 6 min for different initial concentrations. Therefore, 60 min was selected as the optimum contact time for other experiments. When the initial F concentration is increased, the percent of F removal decreased. In contrast, when the initial F concentration is increased, the amounts of adsorbed F also increase. The highest and lowest removal efficiencies were achieved at the initial F concentrations of 5 and 30 mg/L, respectively. As figure 3 reveals, F adsorption capacity by the utilized adsorbent increases as the initial F concentration increases. In the present study, the experimental data of the adsorption equilibrium were investigated using Langmuir and Freundlich isotherm models. The isotherm provides a relationship between the pollutant concentration in solution and the amount of pollutant adsorbed on to the solid phase when both phases are in equilibrium. According to the results, the correlation coefficient of the Langmuir model (R2 = 0.991) was higher than the Freundlich model, indicating that the Langmuir model is suitable for describing F adsorption equilibrium on NaOH -modified Eucalyptus leaves.
Investigation of Adsorption Kinetics
The obtained results indicate that F adsorption on modified eucalyptus complies with the pseudo second-order kinetic model with R2 equal to 0.999.
Conclusion
The results of this study showed that any modification of eucalyptus leaves with
NaOH could lead to surface F adsorption from aqueous solutions with maximum adsorption capacity of 298 mg/g under the optimal adsorption conditions of a pH 2. Initial concentration of 5 mg/L, and the adsorbent dosage of 0.5 g/L, gives a maximum removal efficiency of 97%. It was also documented that the pseudo-second order kinetic model and the Langmuir isotherm model had the best fit with the experimental data, and F adsorption process was facilitated. Eucalyptus modified leaves can be used under optimum conditions to remove fluoride from aqueous solutions.
Acknowledgments
Thanks go to Zahedan University of Medical Sciences for its technical and financial support of this study.
Funding
This study has been supported by the Health Enhancement Research Center of the Zahedan University of Medical Sciences (Project code: 7425).
Conflict of interests
No conflict of interest has been stated by the authors.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use.
Reference
1. Srimurali M, Pragathi A, Karthikeyan J. A study on removal of fluorides from drinking water by adsorption onto low-cost materials. Environmental Pollution. 1998; 99(2): 285-9. doi: https:// doi.org/ 10.1016/S0269-7491(97) 00129- 2.
2. Mahramanlioglu M, Kizilcikli I, Bicer I. Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem. 2002; 115(1): 41-7.
3.WHO. Guidelines for drinking-water quality 2011. 104-8 p. DOI: 10.1016/j. waters. 2007. 04. 029.
4. Mohapatra M, Rout K, Singh P, et al. Fluoride adsorption studies on mixed-phase nano iron oxides prepared by surfactant mediation-precipitation technique. Journal of hazardous materials. 2011; 186(2): 1751-7.
5. De la Puente G, Pis J, Menendez J, et al. Thermal stability of oxygenated functions in activated carbons. J Anal Appl Pyrolysis. 1997; 43(2): 125-38.
6. Ho LN, Ishihara T, Ueshima S, et al. Removal of fluoride from water through ion exchange by mesoporous Ti oxohydroxide. Journal of colloid and interface science. 2004; 272(2): 399-403.
7. Wu X, Zhang Y, Dou X, et al. Fluoride removal performance of a novel Fe–Al–Ce trimetal oxide adsorbent. Chemosphere. 2007; 69(11): 1758-64.
8. Kemer B, Ozdes D, Gundogdu A, et al. Removal of fluoride ions from aqueous solution by waste mud. Journal of hazardous materials. 2009; 168(2): 888-94.
9. Çengeloğlu Y, Kır E, Ersöz M. Removal of fluoride from aqueous solution by using red mud. Sep Purif Technol. 2002; 28(1): 81-6.
10. Fazlzadeh M, Hazrati S, Adhami S. Modification of green clay by HCl and H2SO4 to remove humic acid from aqueous solutions. Journal of Occupational and Environmental Health. 2016; 2(1): 27-46.
11. Dashti Khavidaki H, Aghaie H. Adsorption of thallium (I) ions using eucalyptus leaves powder. CLEAN–Soil, Air, Water. 2013; 41(7): 673-9.
12. Federation WE, Association AP. Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC, USA. 2005.
13. Faraji M, Bazrafshan E, Almasian M, et al. Investigation of fluoride adsorption from aqueous solutions by modified eucalyptus leaves: isotherm and kinetic and thermodynamic studies. Iranian Journal of Health Sciences. 2017; 5(3): 65-77.
14. Avisar D, Primor O, Gozlan I, et al. Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water, Air, & Soil Pollution. 2010; 209(1-4): 439-50.
15. Jonidi-Jafari A, Shirzad-Siboni M, Yang
J-K, et al. Photocatalytic degradation of diazinon with illuminated ZnO–TiO 2 composite. J Taiwan Inst Chem Eng. 2015; 50(10): 100-7.
16. Bazrafshan E, Mostafapour FK, Hosseini AR, et al. Decolorisation of reactive red 120 dye by using single-walled carbon nanotubes in aqueous solutions. Journal of Chemistry. 2013.
17. Zhang G, He Z, Xu W. A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chemical Engineering Journal. 2012; 183: 315-24.
18. Bazrafshan E, Khoshnamvand N, Mahvi AH. fluoride removal from aqueous environments by zncl 2-treated eucalyptus leaves as a natural adsorbent. Fluoride. 2015; 48(4): 196-202.
19. Zazouli MA, Belarak D, Karimnezhad F, et al. Removal of fluoride from aqueous solution by using of adsorption onto modified Lemna minor: Adsorption isotherm and kinetics study. Journal of Mazandaran University of Medical Sciences. 2014; 23(109): 195-204.
20. Samarghandy MR, Hoseinzadeh E, Taghavi M, et al. Biosorption of reactive black 5 from aqueous solution using acid-treated biomass of potato peel waste. Bio Resources. 2011; 6(4): 4840-55.
Type of Study: Original articles |
Subject:
Special
Received: 2017/10/31 | Accepted: 2018/01/20 | Published: 2018/03/14
Received: 2017/10/31 | Accepted: 2018/01/20 | Published: 2018/03/14
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






