Volume 10, Issue 4 (December 2025)
J Environ Health Sustain Dev 2025, 10(4): 2815-2824 |
Back to browse issues page
Ethics code: IR.KMU.REC.1403.413
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dolatabadi M, Ahmadi J, Ahmadzadeh S. Electrocatalytic Oxidation for Removal of COD from Chlorpyrifos Contaminated Wastewater: Optimization and Performance. J Environ Health Sustain Dev 2025; 10 (4) :2815-2824
URL: http://jehsd.ssu.ac.ir/article-1-1012-en.html
URL: http://jehsd.ssu.ac.ir/article-1-1012-en.html
Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran
Full-Text [PDF 759 kb]
(32 Downloads)
| Abstract (HTML) (108 Views)
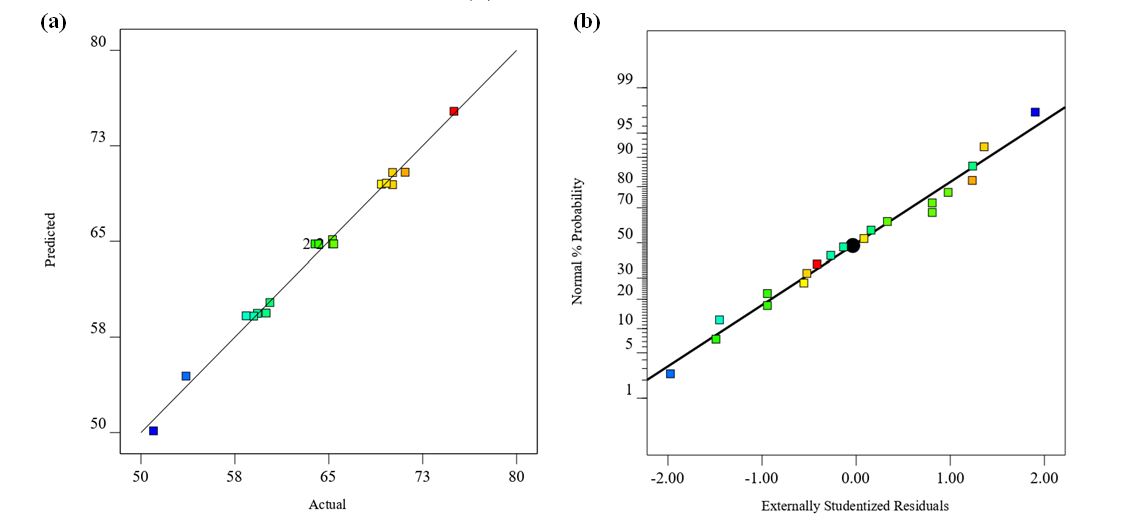
Figure 1: (a) Plot of the actual and predicted values of COD removal efficiency; (b) normal plot of the externally studentized residuals.
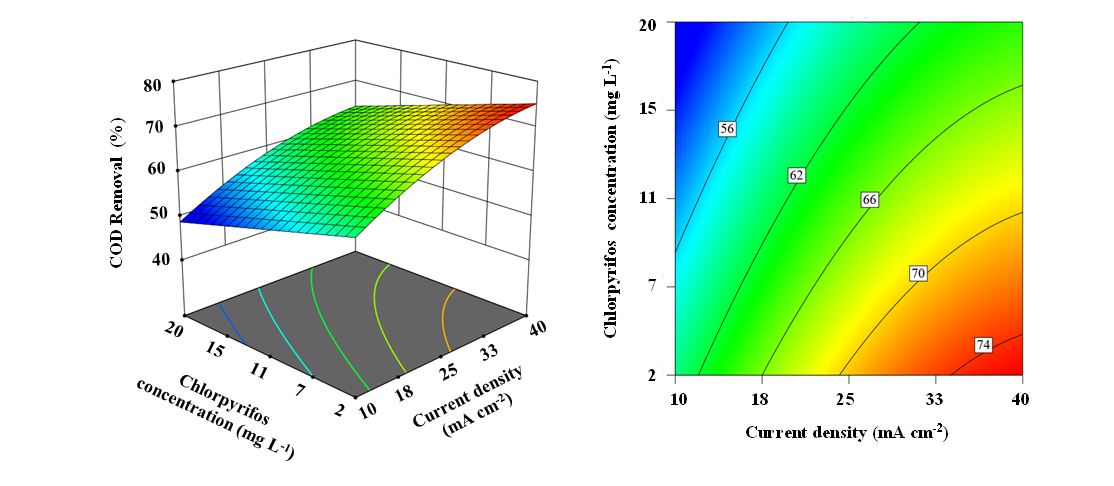
Figure 2:3D and contour plots of CPS concentration and current density for COD removal efficiency.
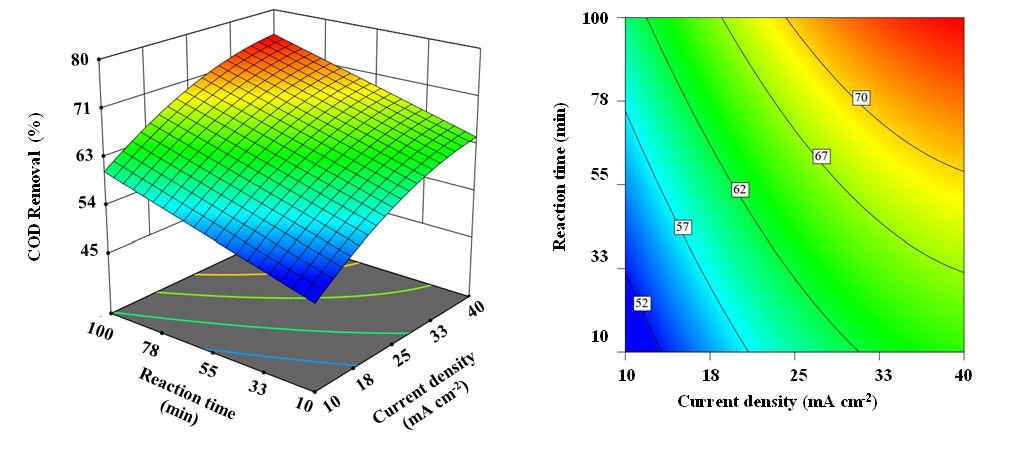
Figure 3: 3D and contour plots of reaction time and current density for COD removal efficiency.
Full-Text: (8 Views)
Electrocatalytic Oxidation for Removal of COD from Chlorpyrifos Contaminated Wastewater: Optimization and Performance
Maryam Dolatabadi 1*, Jafar Ahmadi 2,3, Saeid Ahmadzadeh 4,5
1 Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran.
2 Department of Toxicology and Pharmacology, Kerman University of Medical Sciences, Kerman, Iran.
3 Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran.
4 Pharmaceutics Research Center, Institute of Pharmaceutical Sciences, Kerman University of Medical Sciences, Kerman, Iran.
5 Pharmaceutical Sciences and Cosmetic Products Research Center, Institue of Pharmaceutical Sciences, Kerman University of Medical Sciences, Kerman, Iran.
Maryam Dolatabadi 1*, Jafar Ahmadi 2,3, Saeid Ahmadzadeh 4,5
1 Environmental Health Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran.
2 Department of Toxicology and Pharmacology, Kerman University of Medical Sciences, Kerman, Iran.
3 Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran.
4 Pharmaceutics Research Center, Institute of Pharmaceutical Sciences, Kerman University of Medical Sciences, Kerman, Iran.
5 Pharmaceutical Sciences and Cosmetic Products Research Center, Institue of Pharmaceutical Sciences, Kerman University of Medical Sciences, Kerman, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: The removal of chemical oxygen demand (COD) associated with the pesticide chlorpyrifos (CPS) is critical for protecting aquatic ecosystems and public health. Additionally, incomplete degradation of CPS can yield recalcitrant or more toxic transformation products, thereby increasing ecological and human health risks. Therefore, it is essential to removal the COD contributions attributable to CPS from polluted water. Materials and Methods: In this study, response surface methodology (RSM) was applied as an effective approach to optimize COD removal during anodic oxidation (AO). The effects of the operational parameters, namely CPS concentration, current density, and reaction time, were assessed and subsequently optimized. Results: The model’s predicted COD removal closely matched experimental observations, exhibiting an R2 of 0.9915. ANOVA confirmed the significance of the fitted quadratic model, a high F valuee (324.7), and regression coefficients approaching unity at the 95% confidence level. The lack-of-fit p-value (0.6071) indicates that the lack of fit is not significant. Under optimized conditions, with an CPS concentration of 17.0 mg L⁻¹, current density of 35 mA cm⁻², and a reaction time of 75 min, the maximum COD removal eeficiency reached 70.2%, with an electrical energy consumption of 0.218 kWh m-3. Conclusion: •OH was the predominant oxidizing species mediating the removal of COD associated with CPS during the AO process. The AO process, recognized for its environmental compatibility, was successfully applied for COD removal. The AO process is an effective treatment for pesticide-contaminated wastewater. |
|
Article History: Received: 19 September 2025 Accepted: 20 November 2025 |
||
*Corresponding Author: Maryam Dolatabadi Email: Health.dolatabadi@gmail.com Tel: +98 3431325153 |
||
Keywords: Advanced Oxidation Process; Chemical Oxygen Demand; Chlorpyrifos; Degradation; Removal. |
Citation: Dolatabadi M, Ahmadi J, Ahmadzadeh J. Electrocatalytic Oxidation for Removal of COD from Chlorpyrifos-Contaminated Wastewater: Optimization and Performance. J Environ Health Sustain Dev. 2025; 10(4): 2815-24.
Introduction
Since the beginning of the Green Revolution, contemporary agricultural systems have increasingly relied on the intensive application of agrochemicals, particularly herbicides, insecticides, and fungicides, resulting in their widespread and routine use across modern cropping practices. Pesticides are substances formulated and deployed to prevent, eradicate, repel, or mitigate populations of organisms classified as pests. Among the various classes of pesticides, insecticides (particularly organophosphate compounds) are the most extensively utilized because of their high efficacy and broad-spectrum activity against a wide range of insect pests. Organophosphates constitute approximately 36% of the global pesticide market 1. According to international organizations (such as the FAO and Agriculture Organization), an estimated 500,000 tonnes of obsolete pesticides are currently stockpiled worldwide, of which approximately 200,000 tonnes are organophosphorus compounds 2.
Chlorpyrifos (CPS) is an organophosphorus pesticide widely used to manage a range of arthropod pests in both the larval and adult stages. Its applications target species such as cutworms, dipteran flies, various beetles (including corn rootworms), lice, and fire ants 3. Excessive application of CPS can result in its mobilization into the environment, with documented dispersal distances of up to approximately 24 km from the site of application 4. Research on insects and other pest organisms has shown that CPS binds to the active site of the cholinesterase (ChE) enzyme, inhibiting its catalytic function and thereby obstructing the hydrolysis of acetylcholine (ACh) in the synaptic cleft. Excessive accumulation of ACh in the synaptic cleft provokes prolonged overstimulation of neuronal receptors, culminating in neurotoxic effects and, in severe instances, death 5. The environmental persistence of CPS varies by medium: its half-life in aquatic systems is generally reported to be between 35 and 78 days, whereas in soil, it typically ranges from 60–120 days. Nevertheless, the duration of degradation is highly variable, ranging from approximately two weeks to over one year, depending on factors such as soil composition, temperature, moisture content, microbial activity, and prevailing climatic conditions. Excessive pesticide application adversely affects soil, terrestrial, and aquatic ecosystems through multiple pathways, including accidental spills, surface runoff, over-application, leaching, and container leakage. Consequently, pesticide residues have been documented to exert toxic effects on both human and non-human biota.
CPS exposure has been linked to a range of adverse health outcomes, including an increased risk of childhood leukemia, anemia, renal impairment, and congenital malformations. Evidence indicates that it may induce genomic damage and mutagenesis, such as chromosomal deletions at specific loci. Moreover, CPS and/or its biologically active metabolites may persist across multiple generations, with reports suggesting retention or transgenerational effects lasting at least two generations 2, 6. Moreover, CPS is detrimental to a wide range of nontarget terrestrial organisms, including mammals and avian species. Human epidemiological and experimental studies have associated exposure to adverse outcomes, such as reduced birth weight and impaired neurodevelopment. In vitro investigations have further indicated that CPS may provoke apoptotic pathways in human neuroblastoma cell lines 5, 7.
The extensive agricultural application of CPS, coupled with its persistent residues in environmental matrices, has generated substantial public concern and driven the demand for effective and safe strategies to mitigate pesticide contamination and the associated toxicological risks. Conventional wastewater treatment approaches often suffer from limitations, such as prolonged processing times, limited contaminant degradation efficiency, and the generation of secondary wastewater streams 8. Consequently, advanced oxidation processes (AOPs), including photocatalytic degradation, the Fenton reaction, ozonation, and electrochemical oxidation, have been developed and implemented to address these shortcomings.
Among the suite of advanced oxidation technologies, electrochemical advanced oxidation processes (EAOPs) have attracted substantial interest owing to their ability to produce highly reactive oxidizing species in situ. Specifically, EAOPs produce hydroxyl radicals (•OH) directly at the anode surface, enabling the efficient oxidative decomposition of contaminants. In EAOPs, the choice of electrode material is the principal determinant of its performance. Recently, lead dioxide (PbO2)-based anodes have attracted considerable interest because of their high oxygen evolution potential, favorable cost-effectiveness, and strong capability to generate hydroxyl radicals, which promote efficient contaminant oxidation 9.
This study aimed to assess the effectiveness of anodic oxidation (AO) using a PbO2 anode for the removal of chemical oxygen demand (COD) from synthetic wastewater. The influence of the principal operational parameters, namely, the CPS concentration, applied current density, and reaction duration, on the COD removal efficiency was examined. This study introduces a streamlined, high-efficiency protocol for the removal of COD from CPS-contaminated wastewater. The use of Design‑Expert substantially reduced the number of experimental runs by enabling multivariate optimization and systematic exploration of interaction effects that OFAT approaches cannot capture. The resulting design increased the statistical power, more reliably identified the optimal operating windows, and shortened the development time while reducing the material and energy consumption. Sensitive ICP‑MS monitoring of heavy metal release delivers more robust, reproducible data and clearer, practically actionable guidance for process scale‑up and implementation.
Materials and Methods
Materials and chemicals
Tin chloride, HCl, nitric acid, antimony trichloride, lead nitrate, ethylene glycol, 2-propanol, sodium hydroxide, ammonium acetate, and sodium fluoride were purchased from Merck Millipore Co. Titanium plates (purity ≥ 99.5%) were procured from a local company in Tehran, Iran.
Preparation of electrode
A Ti plate was used as the substrate for fabricating the modified electrodes. To remove surface contaminants, including native oxide films, the Ti electrodes (100 × 20 × 1 mm) were mechanically polished with abrasive paper and subsequently ultrasonically cleaned in ethanol. To remove residual oil from the electrode surface, the titanium plate was immersed in a 40% sodium hydroxide solution and heated at 80 °C for 180 min. Subsequently, the electrode was subjected to etching by immersion in boiling 20% HCl for 60 min. Finally, the specimen was rinsed thoroughly with copious amounts of deionized distilled water (DDW). To enhance the catalytic performance of the electrode, a SnO2–Sb2O3 (Sn/Sb) sublayer was applied to its surface using the dip-coating technique and subsequently thermally deposited, following the procedure described by Yao et al. 10. Subsequently, an intermediate PbO2 layer was electrochemically deposited onto the Sn/Sb sublayer to decrease the electrode–substrate resistance, in accordance with the method reported by Duan et al. 11.
Electrochemical treatment setup and analysis methods
Electrochemical experiments were performed in a 250 mL laboratory reactor using an MP‑3005D Megatek power supply, with agitation provided by a magnetic stirrer (Heidolph). Two electrodes were employed: a stainless-steel plate as the cathode and a fabricated electrode as the anode. Both electrodes possessed identical dimensions, with an immersed surface area of 30 cm² (75 × 20 × 1 mm), and were spaced 2.5 cm apart. A 50 mM Na2SO4 solution was used as the supporting electrolyte for all runs. COD measurements were conducted using the closed-reflux dichromate colorimetric method. Analyses were performed using a UV-Vis spectrophotometer (model OPTIZEN 3220UV, Korea) following the relevant Standard Methods protocol for a COD range of 0-1500 mg O2 L⁻¹.
Design of Experiments
Response surface methodology (RSM) offers a systematic framework for process modeling and enables the efficient exploration of the experimental factor space, requiring far fewer runs than the one-factor-at-a-time method. The process variables were optimized using RSM, with the COD removal efficiency as the response metric. The treatment experiments were designed, and the responses were analyzed using the Design-Expert software. A central composite design (CCD) based on RSM was employed to model the relationships between the process variables and the response and to evaluate the interactions among the variables. The independent variables, namely CPS concentration (2–20 mg L⁻¹), current density (10–40 mA cm⁻²), and reaction time (10–100 min), were encoded at five levels (+α, +1, 0, -1, and -α).
A polynomial model was developed to predict the COD removal efficiency of the AO process. This model was intended to characterize the relationships between the principal operational parameters, which were considered independent variables 12, 13.
+ ε
Y represents the response variable, which consists of the COD removal efficiency. The constant coefficient is denoted as β0, and βi, βii, and βij represent the linear, quadratic, and interaction coefficients, respectively. The number of variables is denoted by n, and ε denotes the model error 14, 15. Significance was assessed at a p-value threshold of ≤ 0.05, corresponding to a 95% confidence level. The experimental validation comprised 20 runs conducted according to the CCD.
Result
Analysis of variance (ANOVA)
RSM was applied as a statistical tool to optimize the treatment process variables while reducing the required number of experimental runs. The resulting COD removal efficiencies, which served as the response values for the developed process, are presented in Table 1.
Since the beginning of the Green Revolution, contemporary agricultural systems have increasingly relied on the intensive application of agrochemicals, particularly herbicides, insecticides, and fungicides, resulting in their widespread and routine use across modern cropping practices. Pesticides are substances formulated and deployed to prevent, eradicate, repel, or mitigate populations of organisms classified as pests. Among the various classes of pesticides, insecticides (particularly organophosphate compounds) are the most extensively utilized because of their high efficacy and broad-spectrum activity against a wide range of insect pests. Organophosphates constitute approximately 36% of the global pesticide market 1. According to international organizations (such as the FAO and Agriculture Organization), an estimated 500,000 tonnes of obsolete pesticides are currently stockpiled worldwide, of which approximately 200,000 tonnes are organophosphorus compounds 2.
Chlorpyrifos (CPS) is an organophosphorus pesticide widely used to manage a range of arthropod pests in both the larval and adult stages. Its applications target species such as cutworms, dipteran flies, various beetles (including corn rootworms), lice, and fire ants 3. Excessive application of CPS can result in its mobilization into the environment, with documented dispersal distances of up to approximately 24 km from the site of application 4. Research on insects and other pest organisms has shown that CPS binds to the active site of the cholinesterase (ChE) enzyme, inhibiting its catalytic function and thereby obstructing the hydrolysis of acetylcholine (ACh) in the synaptic cleft. Excessive accumulation of ACh in the synaptic cleft provokes prolonged overstimulation of neuronal receptors, culminating in neurotoxic effects and, in severe instances, death 5. The environmental persistence of CPS varies by medium: its half-life in aquatic systems is generally reported to be between 35 and 78 days, whereas in soil, it typically ranges from 60–120 days. Nevertheless, the duration of degradation is highly variable, ranging from approximately two weeks to over one year, depending on factors such as soil composition, temperature, moisture content, microbial activity, and prevailing climatic conditions. Excessive pesticide application adversely affects soil, terrestrial, and aquatic ecosystems through multiple pathways, including accidental spills, surface runoff, over-application, leaching, and container leakage. Consequently, pesticide residues have been documented to exert toxic effects on both human and non-human biota.
CPS exposure has been linked to a range of adverse health outcomes, including an increased risk of childhood leukemia, anemia, renal impairment, and congenital malformations. Evidence indicates that it may induce genomic damage and mutagenesis, such as chromosomal deletions at specific loci. Moreover, CPS and/or its biologically active metabolites may persist across multiple generations, with reports suggesting retention or transgenerational effects lasting at least two generations 2, 6. Moreover, CPS is detrimental to a wide range of nontarget terrestrial organisms, including mammals and avian species. Human epidemiological and experimental studies have associated exposure to adverse outcomes, such as reduced birth weight and impaired neurodevelopment. In vitro investigations have further indicated that CPS may provoke apoptotic pathways in human neuroblastoma cell lines 5, 7.
The extensive agricultural application of CPS, coupled with its persistent residues in environmental matrices, has generated substantial public concern and driven the demand for effective and safe strategies to mitigate pesticide contamination and the associated toxicological risks. Conventional wastewater treatment approaches often suffer from limitations, such as prolonged processing times, limited contaminant degradation efficiency, and the generation of secondary wastewater streams 8. Consequently, advanced oxidation processes (AOPs), including photocatalytic degradation, the Fenton reaction, ozonation, and electrochemical oxidation, have been developed and implemented to address these shortcomings.
Among the suite of advanced oxidation technologies, electrochemical advanced oxidation processes (EAOPs) have attracted substantial interest owing to their ability to produce highly reactive oxidizing species in situ. Specifically, EAOPs produce hydroxyl radicals (•OH) directly at the anode surface, enabling the efficient oxidative decomposition of contaminants. In EAOPs, the choice of electrode material is the principal determinant of its performance. Recently, lead dioxide (PbO2)-based anodes have attracted considerable interest because of their high oxygen evolution potential, favorable cost-effectiveness, and strong capability to generate hydroxyl radicals, which promote efficient contaminant oxidation 9.
This study aimed to assess the effectiveness of anodic oxidation (AO) using a PbO2 anode for the removal of chemical oxygen demand (COD) from synthetic wastewater. The influence of the principal operational parameters, namely, the CPS concentration, applied current density, and reaction duration, on the COD removal efficiency was examined. This study introduces a streamlined, high-efficiency protocol for the removal of COD from CPS-contaminated wastewater. The use of Design‑Expert substantially reduced the number of experimental runs by enabling multivariate optimization and systematic exploration of interaction effects that OFAT approaches cannot capture. The resulting design increased the statistical power, more reliably identified the optimal operating windows, and shortened the development time while reducing the material and energy consumption. Sensitive ICP‑MS monitoring of heavy metal release delivers more robust, reproducible data and clearer, practically actionable guidance for process scale‑up and implementation.
Materials and Methods
Materials and chemicals
Tin chloride, HCl, nitric acid, antimony trichloride, lead nitrate, ethylene glycol, 2-propanol, sodium hydroxide, ammonium acetate, and sodium fluoride were purchased from Merck Millipore Co. Titanium plates (purity ≥ 99.5%) were procured from a local company in Tehran, Iran.
Preparation of electrode
A Ti plate was used as the substrate for fabricating the modified electrodes. To remove surface contaminants, including native oxide films, the Ti electrodes (100 × 20 × 1 mm) were mechanically polished with abrasive paper and subsequently ultrasonically cleaned in ethanol. To remove residual oil from the electrode surface, the titanium plate was immersed in a 40% sodium hydroxide solution and heated at 80 °C for 180 min. Subsequently, the electrode was subjected to etching by immersion in boiling 20% HCl for 60 min. Finally, the specimen was rinsed thoroughly with copious amounts of deionized distilled water (DDW). To enhance the catalytic performance of the electrode, a SnO2–Sb2O3 (Sn/Sb) sublayer was applied to its surface using the dip-coating technique and subsequently thermally deposited, following the procedure described by Yao et al. 10. Subsequently, an intermediate PbO2 layer was electrochemically deposited onto the Sn/Sb sublayer to decrease the electrode–substrate resistance, in accordance with the method reported by Duan et al. 11.
Electrochemical treatment setup and analysis methods
Electrochemical experiments were performed in a 250 mL laboratory reactor using an MP‑3005D Megatek power supply, with agitation provided by a magnetic stirrer (Heidolph). Two electrodes were employed: a stainless-steel plate as the cathode and a fabricated electrode as the anode. Both electrodes possessed identical dimensions, with an immersed surface area of 30 cm² (75 × 20 × 1 mm), and were spaced 2.5 cm apart. A 50 mM Na2SO4 solution was used as the supporting electrolyte for all runs. COD measurements were conducted using the closed-reflux dichromate colorimetric method. Analyses were performed using a UV-Vis spectrophotometer (model OPTIZEN 3220UV, Korea) following the relevant Standard Methods protocol for a COD range of 0-1500 mg O2 L⁻¹.
Design of Experiments
Response surface methodology (RSM) offers a systematic framework for process modeling and enables the efficient exploration of the experimental factor space, requiring far fewer runs than the one-factor-at-a-time method. The process variables were optimized using RSM, with the COD removal efficiency as the response metric. The treatment experiments were designed, and the responses were analyzed using the Design-Expert software. A central composite design (CCD) based on RSM was employed to model the relationships between the process variables and the response and to evaluate the interactions among the variables. The independent variables, namely CPS concentration (2–20 mg L⁻¹), current density (10–40 mA cm⁻²), and reaction time (10–100 min), were encoded at five levels (+α, +1, 0, -1, and -α).
A polynomial model was developed to predict the COD removal efficiency of the AO process. This model was intended to characterize the relationships between the principal operational parameters, which were considered independent variables 12, 13.
Y represents the response variable, which consists of the COD removal efficiency. The constant coefficient is denoted as β0, and βi, βii, and βij represent the linear, quadratic, and interaction coefficients, respectively. The number of variables is denoted by n, and ε denotes the model error 14, 15. Significance was assessed at a p-value threshold of ≤ 0.05, corresponding to a 95% confidence level. The experimental validation comprised 20 runs conducted according to the CCD.
Result
Analysis of variance (ANOVA)
RSM was applied as a statistical tool to optimize the treatment process variables while reducing the required number of experimental runs. The resulting COD removal efficiencies, which served as the response values for the developed process, are presented in Table 1.
Table 1: Experimental factors and COD removal efficiencies.
| Run | Coded values | Experimental COD removal (%) |
Predicted COD removal (%) |
||
| X1 (mg L-1) | X2 (mA cm-1) | X3 (min) | |||
| 1 | 0 | 0 | 0 | 64.2 | 63.7 |
| 2 | 0 | 0 | 0 | 64.2 | 64.9 |
| 3 | -1 | -1 | +1 | 65.3 | 66.1 |
| 4 | 0 | 0 | 1.5 | 70.1 | 70.8 |
| 5 | 1.5 | 0 | 0 | 59.0 | 58.4 |
| 6 | +1 | -1 | +1 | 59.3 | 58.7 |
| 7 | +1 | +1 | -1 | 60.3 | 60.0 |
| 8 | 0 | 0 | 0 | 63.9 | 62.3 |
| 9 | 0 | 0 | 0 | 65.4 | 65.1 |
| 10 | 0 | -1 | 0 | 53.6 | 54.3 |
| 11 | -1 | +1 | -1 | 69.2 | 68.4 |
| 12 | +1 | -1 | -1 | 51.0 | 50.2 |
| 13 | +1 | +1 | +1 | 70.1 | 69.6 |
| 14 | 0 | 1.5 | 0 | 69.6 | 69.0 |
| 15 | 0 | 0 | 0 | 65.3 | 53.2 |
| 16 | 0 | 0 | 0 | 65.3 | 64.8 |
| 17 | -1 | -1 | -1 | 60.0 | 59.7 |
| 18 | 0 | 0 | -1 | 58.4 | 58.0 |
| 19 | -1 | +1 | +1 | 75.0 | 74.8 |
| 20 | -1 | 0 | 0 | 71.1 | 71.5 |
ANOVA is a statistical method that partitions the total variability in a dataset into component sources of variation associated with specified factors, enabling hypothesis testing of the model variables. The results of ANOVA are presented in Table 2. The p-values for all regression terms were below 0.01, indicating that at least one predictor in the regression equation was significantly associated with the response variable. The ANOVA table also includes a residual error term, which quantifies the portion of variability in the response data that remains unexplained by the model.
Table 2: ANOVA results for the developed models.
| Source | Sum of squares | df | Mean square | F-value | p-value |
| Model | 690.69 | 5 | 138.14 | 324.77 | < 0.0001 |
| X1 | 176.34 | 1 | 176.34 | 414.59 | < 0.0001 |
| X2 | 317.52 | 1 | 317.52 | 746.50 | < 0.0001 |
| X3 | 174.84 | 1 | 174.84 | 411.07 | < 0.0001 |
| X1 X3 | 6.13 | 1 | 6.13 | 14.40 | 0.0020 |
| X22 | 15.86 | 1 | 15.86 | 37.28 | < 0.0001 |
| Residual | 5.95 | 14 | 0.43 | --- | --- |
| Lack of Fit | 3.61 | 9 | 0.40 | 0.85 | 0.6071 |
| Pure Error | 2.35 | 5 | 0.47 | --- | --- |
| Cor Total | 696.65 | 19 | --- | --- | --- |
The goodness of fit of the proposed model was evaluated using the coefficients of determination. The coefficient of determination (R2), adjusted R2 (Adj.R2), and predicted R2 (Pred.R2) for the COD removal efficiency were 0.9915, 0.9884, and 0.9815, respectively.
Based on the model fit assessment, the combination of a very low p-value and a high R2 indicates that the second-order (quadratic) regression model most accurately predicts the COD removal efficiencies. The quadratic regression model developed in this study is as follows:
In this study, Y denotes COD removal efficiency. The coded variables X1, X2, and X3 represent the CPS concentration, current density, and reaction time, respectively. In the fitted regression model, a positive coefficient signifies a synergistic interaction between the factors, whereas a negative coefficient indicates an antagonistic interaction. Figure 1-a compares the observed and predicted values of the COD removal efficiency, illustrating the predictive accuracy of the model. Figure 1-b plots the residuals against the externally studentized residuals, revealing the pattern and distribution of the deviations of the data from the model.
Based on the model fit assessment, the combination of a very low p-value and a high R2 indicates that the second-order (quadratic) regression model most accurately predicts the COD removal efficiencies. The quadratic regression model developed in this study is as follows:
In this study, Y denotes COD removal efficiency. The coded variables X1, X2, and X3 represent the CPS concentration, current density, and reaction time, respectively. In the fitted regression model, a positive coefficient signifies a synergistic interaction between the factors, whereas a negative coefficient indicates an antagonistic interaction. Figure 1-a compares the observed and predicted values of the COD removal efficiency, illustrating the predictive accuracy of the model. Figure 1-b plots the residuals against the externally studentized residuals, revealing the pattern and distribution of the deviations of the data from the model.

Figure 1: (a) Plot of the actual and predicted values of COD removal efficiency; (b) normal plot of the externally studentized residuals.
Effect of parameters on the COD removal efficiency
The influence of the CPS concentration and applied current density on the COD removal efficiency was examined. The results of this investigation are presented graphically as a three-dimensional surface plot in Figure 2.
The influence of the CPS concentration and applied current density on the COD removal efficiency was examined. The results of this investigation are presented graphically as a three-dimensional surface plot in Figure 2.

Figure 2:3D and contour plots of CPS concentration and current density for COD removal efficiency.
The increase in CPS concentration from 2.0 mg L⁻¹ to 20.0 mg L⁻¹ resulted in a marked decline in COD removal efficiency, decreasing from 70.4% to 59.1%.
These measurements were conducted with the applied current density and oxidation duration maintained at their respective central-point values. The results shown in Figure 2 demonstrate a pronounced improvement in COD removal efficiency from 54.4% to 69.5% when the current density was increased from 10 to 40 mA cm⁻². Other parameters were set at their central point values.
The combined effects of reaction time and current density on COD removal efficiency were also investigated. The results of this analysis are presented as a 3D plot in Figure 3. As shown, the COD removal efficiency increased markedly from 59.1% to 70.3% when the oxidation time was extended from 10 to 100 min.
These measurements were conducted with the applied current density and oxidation duration maintained at their respective central-point values. The results shown in Figure 2 demonstrate a pronounced improvement in COD removal efficiency from 54.4% to 69.5% when the current density was increased from 10 to 40 mA cm⁻². Other parameters were set at their central point values.
The combined effects of reaction time and current density on COD removal efficiency were also investigated. The results of this analysis are presented as a 3D plot in Figure 3. As shown, the COD removal efficiency increased markedly from 59.1% to 70.3% when the oxidation time was extended from 10 to 100 min.

Figure 3: 3D and contour plots of reaction time and current density for COD removal efficiency.
Optimization of COD removal using AO
The operational parameters were confined to predefined in-range settings, and the model determined the optimal treatment condition by maximizing the desirability index, which ranged from 0 to 1. Based on the strategies recommended by the developed model, the treatment condition that maximized the removal efficiency was selected as the optimal approach, particularly for the remediation of water containing CPS. The highest COD removal efficiency, conditions an CPS concentration of 17.0 mg L⁻¹, a current density of 35 mA cm⁻², and a reaction time of 75 min the maximum COD removal efficiency achieved was 70.2%.
Safety evaluation of modified electrodes
One major drawback of the AO process is the leaching of toxic ions from the electrodes. ICP-MS was used to assess the safety of the treated aqueous solutions by quantifying Ti, Sb, Sn, and Pb ions following oxidation for 1–5 h. During all experiments, the current density and pH were maintained constant at 35 mA cm⁻² and 6.5 ± 0.2, respectively.
Discussion
Statistical analysis
The findings suggest that when the disparity between the adjusted R² and the predicted R² is under 0.20, the two metrics are considered to exhibit an acceptable level of agreement. Model precision, as assessed by the adequate precision (signal-to-noise ratio), was 70.2, substantially exceeding the recommended minimum threshold of 4 16, 17. Additionally, to evaluate the model's adequacy, the p-value for the lack of fit, which quantifies the extent to which the model fails to capture the variation in the observed data, was employed. The results indicated that the response exhibited a statistically significant lack of fit, implying that the model adequately captured the observed data. This finding indicates that the developed model was statistically significant and provided a reliable representation of the relationship between the principal operating parameters and COD removal efficiencies at the 95% confidence level. The plot in Figure 1-a shows that the residuals largely lie along a straight line, indicating that the errors are approximately normally distributed, thereby supporting the adequacy of the least-squares fit. Additionally, the residual plots in Figure 1-b exhibit no discernible pattern or anomalous structure and display approximately symmetric scatter. Collectively, these diagnostics indicate that the proposed model is adequate, with no evident violation of the assumptions of independence or homoscedasticity (constant variance).
Effect of significant parameters
As observed, for elevated pollutant concentrations, the highest removal efficiencies occurred during the initial stages of the process, followed by a steady decline in efficiency as the experiment progressed. This behavior is characteristic of the electrochemical oxidation of wastewater using anode. This finding indicates that the oxidation rate increases proportionally with the concentration of organic matter. This is commonly attributed to mass transfer limitations, under the assumption that the predominant mechanisms of anodic electrochemical oxidation are either direct electron transfer at the electrode surface or oxidation mediated by hydroxyl radicals.
The current density is a critical parameter in electrocatalytic processes because it dictates the rate of electron transfer and, consequently, controls the production of hydroxyl radicals. An increase in the current density resulted in elevated hydroxyl radical generation, which consequently improved the removal efficiency. However, it is important to acknowledge that this enhancement is not universally advantageous. This phenomenon can be ascribed to the enhanced generation of hydroxyl radicals (•OH) via electrochemical water discharge as the current density increases (see reaction I). An increased current density promotes the electrogeneration of oxidizing •OH species.
Excessively high current densities can cause electrode degradation and increase energy dissipation, ultimately impairing the overall performance. Moreover, elevated current densities can promote the oxygen evolution reaction and water electrolysis, processes that are detrimental to the treatment by diverting charge from pollutant oxidation and reducing the overall process efficiency 18, 19.
Reaction time is critical in AO processes because it determines the pollutant exposure to reactive species, governing the degradation extent and mineralization. Optimization balances the treatment efficacy, selectivity, and operational cost. In this study, the influence of reaction time was examined over 10–100 min. The results demonstrated that the COD removal efficiency increased with longer reaction durations, indicating a positive correlation between exposure time and contaminant degradation. Prolonging the oxidation period led to an increased formation of hydroxyl radicals, which in turn accelerated the reaction kinetics and improved the overall treatment efficacy. However, an excessively prolonged oxidation period leads to substantially higher energy consumption without a commensurate improvement in the COD removal rate 20-22.
Saini et al. examined the optimization of CPS degradation by Fenton oxidation using a central composite design (CCD) and an adaptive neuro-fuzzy inference system (ANFIS). Under the optimized conditions (initial pH 3, H2O2 concentration 0.571 M, and Fe2+ concentration 3 g L−1), they reported maximum CPS removal efficiencies of 78.6%, 94.0%, and 89.34%, respectively, which corresponded to COD reductions of 68.94%, 83.51%, and 74.44%, respectively 23. Youssef et al. investigated the AO process of CPS employing lead dioxide anodes. A maximum COD removal of 76% was obtained under the following conditions: an applied current density of 50 mA cm−2, an initial COD of 450 mg O2 L−1, an electrolysis temperature of 70 °C, and an electrolysis time of 10 h 24. Augustine Chioma et al. evaluated how operating parameters in a UV–Fenton pretreatment, followed by an aerobic sequencing batch reactor, affect the degradation and biodegradability (BOD5/COD ratio) of wastewater spiked with CPS, cypermethrin, and chlorothalonil. The optimal pretreatment conditions were an H2O2/COD molar ratio of 2.0, H2O2/Fe2+ molar ratio of 25, pH 3, and a reaction time of 60 min. Under these conditions, the pretreatment produced COD and TOC reductions of 64.8% and 45.9%, respectively 25.
Cost–benefit strategy
The cost–benefit strategy is a critical tool in scientific and commercial decision-making, enabling a systematic comparison of the economic, environmental, and social impacts of interventions. By quantifying capital, operational, and opportunity costs against measurable and non-market benefits, such as pollution reduction, health risk mitigation, and regulatory compliance, guides prioritization, funding allocation, and policy recommendations. Robust sensitivity and uncertainty analyses and transparent assumptions ensured reproducibility and strengthened the credibility of the conclusions for stakeholders and peer review. A cost–benefit assessment of the use of electrocatalytic oxidation to remove organic pollution from CPS-contaminated wastewater compares the money spent with the value gained. Costs include the purchase and maintenance of equipment, replacement use, and electrodes, as well as indirect expenses. Benefits include cleaner water, lower fines or compliance costs, potential reuse of treated water, and public health and environmental improvements that are harder to price but are important. It is useful to test how sensitive the outcomes are to key factors such as energy prices and electrode lifespan to determine when the method becomes economical. Comparing these results with those of alternative treatment options can help determine whether electrocatalytic oxidation is a cost-effective choice.
Conclusion
In this study, a CCD was implemented to optimize COD removal via the AO process. The main variables examined during optimization were the CPS concentration, applied current density, and reaction time. Under the optimized operational conditions, a maximum COD removal efficiency of 70.2% was achieved. The statistical analysis indicated a strong agreement between the model predictions and experimental results, as evidenced by a p-value < 0.0001, an F-value of 324.7, a high R2 of 0.9915, and an adj. R2 of 0.9884, and a pred. R2 of 0.9815. ICP‑MS analysis showed that none of the targeted toxic heavy metals were detectable in the treated aqueous samples after 1–5 h of oxidation. The detailed mechanism of the AO process indicated that the removal was predominantly by hydroxyl radicals.
Acknowledgments
The authors would like to express their appreciation to Kerman University of Medical Sciences [Grant number 403000811] for supporting the current work.
Conflict of interest
The authors declare that they have no conflicts of interest regarding the publication of the current paper.
Funding
This work was supported by a grant from Kerman University of Medical Sciences [Grant number 403000811].
Ethical Considerations
The present study is an original laboratory-based (in vitro) investigation and did not involve human or animal subjects; therefore, ethical approval and informed consent were not applicable.
Code of Ethics
The current study was approved by the Ethics Committee of Kerman University of Medical Sciences. The Ethics Approval Code is IR.KMU.REC.1403.413.
Authors' Contributions
M.D.: designed the model and conducted the experiments. J. A. and M.D. contributed to the interpretation of the results and development of the method. S. A. and M.D.: conceived the original idea, supervised the project, and prepared the manuscript. All authors have discussed the results and contributed to the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Jacob MM, Ponnuchamy M, Kapoor A, et al. Achieving up to 95% removal efficiency of chlorpyrifos pesticide using sugarcane bagasse-based biochar alginate beads in a continuous fixed-bed adsorption column. Environ Res. 2025;269:120902.
2. Hamad MTMH, Mahran BNA. Biosynthesized CS-MgO/Zeolite hybrid material: an efficient adsorbent for chlorpyrifos removal - Kinetic studies and response surface methodology. Emerg Contam. 2024;10(3):100324.
3. Dai W, Luo P, Xu B, et al. Efficient removal of chlorpyrifos from water using acrylic resin P(MA-SMA-St): adsorption behavior, kinetics, thermodynamics, molecular simulations, and DFT calculations. J Environ Chem Eng. 2025;13(2):115924.
4. Prabhakar N, Isloor AM, Farnood R. Effective removal of hazardous atrazine and chlorpyrifos by waste PET bottles-derived linker having novel MIL-53(Al)/PMMA-nanofiber incorporated poly(vinylidene) fluoride membranes. J Environ Chem Eng. 2025; 13(2):115351.
5. Singh M, Rano S, Roy S, et al. Characterization of organophosphate pesticide sorption of potato peel biochar as low cost adsorbent for chlorpyrifos removal. Chemosphere. 2022;297: 134112.
6. Jalili M, Abbasi F, Dalvand A, et al. The ozonation effect on flocculation and polymer consumption reduction in the hybrid treatment of Iran Central iron ore companies' effluent: a cost–benefit study. Appl Water Sci. 2023;13(2):51.
7. Salehi H, Ebrahimi AA, Ehrampoush MH, et al. Integration of photo-oxidation based on UV/Persulfate and adsorption processes for arsenic removal from aqueous solutions. Groundw Sustain Dev. 2020;10:100338.
8. Rezayi M, Kassim A, Ahmadzadeh S, et al. Conductometric determination of formation constants of tris (2-pyridyl) methylamine and titanium (III) in water-acetonitryl mixture. Int J Electrochem Sci. 2011;6(9):4378-87.
9. Yu B, Xu R, Wang X, et al. Electrodeposition of Pb-0.6 %Sb/α-PbO2/β-PbO2-CNTs electrodes: Investigation focusing on co-deposition modification mechanism and energy consumption. Int J Electrochem Sci. 2024;19(8):100679.
10. Yao Y, Zhao M, Zhao C, et al. Preparation and properties of PbO2-ZrO2 nanocomposite electrodes by pulse electrodeposition. Electrochim Acta. 2014;117:453-9.
11. Duan X, Ma F, Yuan Z, et al. Lauryl benzene sulfonic acid sodium-carbon nanotube-modified PbO2 electrode for the degradation of 4-chlorophenol. Electrochim Acta. 2012;76:333-43.
12. Zhang L, Wang Y, Liu Y, et al. Chloride removal from sewage using bismuth trioxide: characterization and optimization by response surface methodology (RSM). J Environ Chem Eng. 2023;11(5):110868.
13. Prerana K, Leela Prasanna D, Rahul Kumar J, et al. The significance of synthesized marine algae/ZnO nanoparticles for the degradation of sodium do-decyl sulphate: Response surface methodology. Mater Today Proc. 2023.
14. Asaad SM, Inayat A, Ghenai C, et al. Response surface methodology in biodiesel production and engine performance assessment. International Journal of Thermofluids. 2024:100551.
15. Liu G, Luo D, Wang L, et al. Current status and future perspective in electro-Fenton techniques for wastewater treatment: a bibliometric review. Appl Nanosci. 2023;13(9): 5885-902.
16. Sarip MSM, Lyna FKS, Mansor MR, et al. Modelling of the rice bran protein extraction using response surface methodology. InComputer Aided Chemical Engineering. Elsevier; 2022; 49:p. 823-8.
17. Mujtaba S, Bibi I, Majid F, et al. Optical, dielectric and photocatalytic properties of Ag-doped Sr1−xAgxFe12O19 and CV dye degradation under visible light irradiation. J Alloys Compd. 2024;972:172793.
18. Muchuweni E, Mombeshora ET, Muiva CM, et al. Lithium-ion batteries: recent progress in improving the cycling and rate performances of transition metal oxide anodes by incorporating graphene-based materials. J Energy Storage. 2023;73:109013.
19. Roestamy M, Martin AY, Rusli RK, et al. A review of the reliability of land bank institution in Indonesia for effective land management of public interest. Land use policy. 2022;120: 106275.
20. Hu Z, Cai J, Song G, et al. Anodic oxidation of organic pollutants: anode fabrication, process hybrid and environmental applications. Curr Opin Electrochem. 2021;26:100659.
21. Sheikh MC, Hasan MM, Hasan MN, et al. Toxic cadmium(II) monitoring and removal from aqueous solution using ligand-based facial composite adsorbent. J Mol Liq. 2023;389:122854.
22. Yoosefian M, Ayoubi E, Atanase LI. Palladium-doped single-walled carbon nanotubes as a new adsorbent for detecting and trapping volatile organic compounds: a first principle study. Nanomaterials. 2022;12(15): 2572.
23. Saini R, Kumar P. Optimization of chlorpyrifos degradation by Fenton oxidation using CCD and ANFIS computing technique. J Environ Chem Eng. 2016;4(3):2952-63.
24. Samet Y, Agengui L, Abdelhédi R. Anodic oxidation of chlorpyrifos in aqueous solution at lead dioxide electrodes. Journal of Electroanalytical Chemistry. 2010;650(1):152-8.
25. Affam AC, Chaudhuri M, Kutty SRM, et al. UV Fenton and sequencing batch reactor treatment of chlorpyrifos, cypermethrin and chlorothalonil pesticide wastewater. Int Biodeterior Biodegradation. 2014;93: 195-201.
The operational parameters were confined to predefined in-range settings, and the model determined the optimal treatment condition by maximizing the desirability index, which ranged from 0 to 1. Based on the strategies recommended by the developed model, the treatment condition that maximized the removal efficiency was selected as the optimal approach, particularly for the remediation of water containing CPS. The highest COD removal efficiency, conditions an CPS concentration of 17.0 mg L⁻¹, a current density of 35 mA cm⁻², and a reaction time of 75 min the maximum COD removal efficiency achieved was 70.2%.
Safety evaluation of modified electrodes
One major drawback of the AO process is the leaching of toxic ions from the electrodes. ICP-MS was used to assess the safety of the treated aqueous solutions by quantifying Ti, Sb, Sn, and Pb ions following oxidation for 1–5 h. During all experiments, the current density and pH were maintained constant at 35 mA cm⁻² and 6.5 ± 0.2, respectively.
Discussion
Statistical analysis
The findings suggest that when the disparity between the adjusted R² and the predicted R² is under 0.20, the two metrics are considered to exhibit an acceptable level of agreement. Model precision, as assessed by the adequate precision (signal-to-noise ratio), was 70.2, substantially exceeding the recommended minimum threshold of 4 16, 17. Additionally, to evaluate the model's adequacy, the p-value for the lack of fit, which quantifies the extent to which the model fails to capture the variation in the observed data, was employed. The results indicated that the response exhibited a statistically significant lack of fit, implying that the model adequately captured the observed data. This finding indicates that the developed model was statistically significant and provided a reliable representation of the relationship between the principal operating parameters and COD removal efficiencies at the 95% confidence level. The plot in Figure 1-a shows that the residuals largely lie along a straight line, indicating that the errors are approximately normally distributed, thereby supporting the adequacy of the least-squares fit. Additionally, the residual plots in Figure 1-b exhibit no discernible pattern or anomalous structure and display approximately symmetric scatter. Collectively, these diagnostics indicate that the proposed model is adequate, with no evident violation of the assumptions of independence or homoscedasticity (constant variance).
Effect of significant parameters
As observed, for elevated pollutant concentrations, the highest removal efficiencies occurred during the initial stages of the process, followed by a steady decline in efficiency as the experiment progressed. This behavior is characteristic of the electrochemical oxidation of wastewater using anode. This finding indicates that the oxidation rate increases proportionally with the concentration of organic matter. This is commonly attributed to mass transfer limitations, under the assumption that the predominant mechanisms of anodic electrochemical oxidation are either direct electron transfer at the electrode surface or oxidation mediated by hydroxyl radicals.
The current density is a critical parameter in electrocatalytic processes because it dictates the rate of electron transfer and, consequently, controls the production of hydroxyl radicals. An increase in the current density resulted in elevated hydroxyl radical generation, which consequently improved the removal efficiency. However, it is important to acknowledge that this enhancement is not universally advantageous. This phenomenon can be ascribed to the enhanced generation of hydroxyl radicals (•OH) via electrochemical water discharge as the current density increases (see reaction I). An increased current density promotes the electrogeneration of oxidizing •OH species.
Excessively high current densities can cause electrode degradation and increase energy dissipation, ultimately impairing the overall performance. Moreover, elevated current densities can promote the oxygen evolution reaction and water electrolysis, processes that are detrimental to the treatment by diverting charge from pollutant oxidation and reducing the overall process efficiency 18, 19.
Reaction time is critical in AO processes because it determines the pollutant exposure to reactive species, governing the degradation extent and mineralization. Optimization balances the treatment efficacy, selectivity, and operational cost. In this study, the influence of reaction time was examined over 10–100 min. The results demonstrated that the COD removal efficiency increased with longer reaction durations, indicating a positive correlation between exposure time and contaminant degradation. Prolonging the oxidation period led to an increased formation of hydroxyl radicals, which in turn accelerated the reaction kinetics and improved the overall treatment efficacy. However, an excessively prolonged oxidation period leads to substantially higher energy consumption without a commensurate improvement in the COD removal rate 20-22.
Saini et al. examined the optimization of CPS degradation by Fenton oxidation using a central composite design (CCD) and an adaptive neuro-fuzzy inference system (ANFIS). Under the optimized conditions (initial pH 3, H2O2 concentration 0.571 M, and Fe2+ concentration 3 g L−1), they reported maximum CPS removal efficiencies of 78.6%, 94.0%, and 89.34%, respectively, which corresponded to COD reductions of 68.94%, 83.51%, and 74.44%, respectively 23. Youssef et al. investigated the AO process of CPS employing lead dioxide anodes. A maximum COD removal of 76% was obtained under the following conditions: an applied current density of 50 mA cm−2, an initial COD of 450 mg O2 L−1, an electrolysis temperature of 70 °C, and an electrolysis time of 10 h 24. Augustine Chioma et al. evaluated how operating parameters in a UV–Fenton pretreatment, followed by an aerobic sequencing batch reactor, affect the degradation and biodegradability (BOD5/COD ratio) of wastewater spiked with CPS, cypermethrin, and chlorothalonil. The optimal pretreatment conditions were an H2O2/COD molar ratio of 2.0, H2O2/Fe2+ molar ratio of 25, pH 3, and a reaction time of 60 min. Under these conditions, the pretreatment produced COD and TOC reductions of 64.8% and 45.9%, respectively 25.
Cost–benefit strategy
The cost–benefit strategy is a critical tool in scientific and commercial decision-making, enabling a systematic comparison of the economic, environmental, and social impacts of interventions. By quantifying capital, operational, and opportunity costs against measurable and non-market benefits, such as pollution reduction, health risk mitigation, and regulatory compliance, guides prioritization, funding allocation, and policy recommendations. Robust sensitivity and uncertainty analyses and transparent assumptions ensured reproducibility and strengthened the credibility of the conclusions for stakeholders and peer review. A cost–benefit assessment of the use of electrocatalytic oxidation to remove organic pollution from CPS-contaminated wastewater compares the money spent with the value gained. Costs include the purchase and maintenance of equipment, replacement use, and electrodes, as well as indirect expenses. Benefits include cleaner water, lower fines or compliance costs, potential reuse of treated water, and public health and environmental improvements that are harder to price but are important. It is useful to test how sensitive the outcomes are to key factors such as energy prices and electrode lifespan to determine when the method becomes economical. Comparing these results with those of alternative treatment options can help determine whether electrocatalytic oxidation is a cost-effective choice.
Conclusion
In this study, a CCD was implemented to optimize COD removal via the AO process. The main variables examined during optimization were the CPS concentration, applied current density, and reaction time. Under the optimized operational conditions, a maximum COD removal efficiency of 70.2% was achieved. The statistical analysis indicated a strong agreement between the model predictions and experimental results, as evidenced by a p-value < 0.0001, an F-value of 324.7, a high R2 of 0.9915, and an adj. R2 of 0.9884, and a pred. R2 of 0.9815. ICP‑MS analysis showed that none of the targeted toxic heavy metals were detectable in the treated aqueous samples after 1–5 h of oxidation. The detailed mechanism of the AO process indicated that the removal was predominantly by hydroxyl radicals.
Acknowledgments
The authors would like to express their appreciation to Kerman University of Medical Sciences [Grant number 403000811] for supporting the current work.
Conflict of interest
The authors declare that they have no conflicts of interest regarding the publication of the current paper.
Funding
This work was supported by a grant from Kerman University of Medical Sciences [Grant number 403000811].
Ethical Considerations
The present study is an original laboratory-based (in vitro) investigation and did not involve human or animal subjects; therefore, ethical approval and informed consent were not applicable.
Code of Ethics
The current study was approved by the Ethics Committee of Kerman University of Medical Sciences. The Ethics Approval Code is IR.KMU.REC.1403.413.
Authors' Contributions
M.D.: designed the model and conducted the experiments. J. A. and M.D. contributed to the interpretation of the results and development of the method. S. A. and M.D.: conceived the original idea, supervised the project, and prepared the manuscript. All authors have discussed the results and contributed to the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Jacob MM, Ponnuchamy M, Kapoor A, et al. Achieving up to 95% removal efficiency of chlorpyrifos pesticide using sugarcane bagasse-based biochar alginate beads in a continuous fixed-bed adsorption column. Environ Res. 2025;269:120902.
2. Hamad MTMH, Mahran BNA. Biosynthesized CS-MgO/Zeolite hybrid material: an efficient adsorbent for chlorpyrifos removal - Kinetic studies and response surface methodology. Emerg Contam. 2024;10(3):100324.
3. Dai W, Luo P, Xu B, et al. Efficient removal of chlorpyrifos from water using acrylic resin P(MA-SMA-St): adsorption behavior, kinetics, thermodynamics, molecular simulations, and DFT calculations. J Environ Chem Eng. 2025;13(2):115924.
4. Prabhakar N, Isloor AM, Farnood R. Effective removal of hazardous atrazine and chlorpyrifos by waste PET bottles-derived linker having novel MIL-53(Al)/PMMA-nanofiber incorporated poly(vinylidene) fluoride membranes. J Environ Chem Eng. 2025; 13(2):115351.
5. Singh M, Rano S, Roy S, et al. Characterization of organophosphate pesticide sorption of potato peel biochar as low cost adsorbent for chlorpyrifos removal. Chemosphere. 2022;297: 134112.
6. Jalili M, Abbasi F, Dalvand A, et al. The ozonation effect on flocculation and polymer consumption reduction in the hybrid treatment of Iran Central iron ore companies' effluent: a cost–benefit study. Appl Water Sci. 2023;13(2):51.
7. Salehi H, Ebrahimi AA, Ehrampoush MH, et al. Integration of photo-oxidation based on UV/Persulfate and adsorption processes for arsenic removal from aqueous solutions. Groundw Sustain Dev. 2020;10:100338.
8. Rezayi M, Kassim A, Ahmadzadeh S, et al. Conductometric determination of formation constants of tris (2-pyridyl) methylamine and titanium (III) in water-acetonitryl mixture. Int J Electrochem Sci. 2011;6(9):4378-87.
9. Yu B, Xu R, Wang X, et al. Electrodeposition of Pb-0.6 %Sb/α-PbO2/β-PbO2-CNTs electrodes: Investigation focusing on co-deposition modification mechanism and energy consumption. Int J Electrochem Sci. 2024;19(8):100679.
10. Yao Y, Zhao M, Zhao C, et al. Preparation and properties of PbO2-ZrO2 nanocomposite electrodes by pulse electrodeposition. Electrochim Acta. 2014;117:453-9.
11. Duan X, Ma F, Yuan Z, et al. Lauryl benzene sulfonic acid sodium-carbon nanotube-modified PbO2 electrode for the degradation of 4-chlorophenol. Electrochim Acta. 2012;76:333-43.
12. Zhang L, Wang Y, Liu Y, et al. Chloride removal from sewage using bismuth trioxide: characterization and optimization by response surface methodology (RSM). J Environ Chem Eng. 2023;11(5):110868.
13. Prerana K, Leela Prasanna D, Rahul Kumar J, et al. The significance of synthesized marine algae/ZnO nanoparticles for the degradation of sodium do-decyl sulphate: Response surface methodology. Mater Today Proc. 2023.
14. Asaad SM, Inayat A, Ghenai C, et al. Response surface methodology in biodiesel production and engine performance assessment. International Journal of Thermofluids. 2024:100551.
15. Liu G, Luo D, Wang L, et al. Current status and future perspective in electro-Fenton techniques for wastewater treatment: a bibliometric review. Appl Nanosci. 2023;13(9): 5885-902.
16. Sarip MSM, Lyna FKS, Mansor MR, et al. Modelling of the rice bran protein extraction using response surface methodology. InComputer Aided Chemical Engineering. Elsevier; 2022; 49:p. 823-8.
17. Mujtaba S, Bibi I, Majid F, et al. Optical, dielectric and photocatalytic properties of Ag-doped Sr1−xAgxFe12O19 and CV dye degradation under visible light irradiation. J Alloys Compd. 2024;972:172793.
18. Muchuweni E, Mombeshora ET, Muiva CM, et al. Lithium-ion batteries: recent progress in improving the cycling and rate performances of transition metal oxide anodes by incorporating graphene-based materials. J Energy Storage. 2023;73:109013.
19. Roestamy M, Martin AY, Rusli RK, et al. A review of the reliability of land bank institution in Indonesia for effective land management of public interest. Land use policy. 2022;120: 106275.
20. Hu Z, Cai J, Song G, et al. Anodic oxidation of organic pollutants: anode fabrication, process hybrid and environmental applications. Curr Opin Electrochem. 2021;26:100659.
21. Sheikh MC, Hasan MM, Hasan MN, et al. Toxic cadmium(II) monitoring and removal from aqueous solution using ligand-based facial composite adsorbent. J Mol Liq. 2023;389:122854.
22. Yoosefian M, Ayoubi E, Atanase LI. Palladium-doped single-walled carbon nanotubes as a new adsorbent for detecting and trapping volatile organic compounds: a first principle study. Nanomaterials. 2022;12(15): 2572.
23. Saini R, Kumar P. Optimization of chlorpyrifos degradation by Fenton oxidation using CCD and ANFIS computing technique. J Environ Chem Eng. 2016;4(3):2952-63.
24. Samet Y, Agengui L, Abdelhédi R. Anodic oxidation of chlorpyrifos in aqueous solution at lead dioxide electrodes. Journal of Electroanalytical Chemistry. 2010;650(1):152-8.
25. Affam AC, Chaudhuri M, Kutty SRM, et al. UV Fenton and sequencing batch reactor treatment of chlorpyrifos, cypermethrin and chlorothalonil pesticide wastewater. Int Biodeterior Biodegradation. 2014;93: 195-201.
Type of Study: Original articles |
Subject:
Water quality and wastewater treatment and reuse
Received: 2025/09/19 | Accepted: 2025/11/20 | Published: 2025/12/25
Received: 2025/09/19 | Accepted: 2025/11/20 | Published: 2025/12/25
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







