Volume 10, Issue 3 (September 2025)
J Environ Health Sustain Dev 2025, 10(3): 2771-2780 |
Back to browse issues page
Ethics code: IR.RUMS.REC.1403.087
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Isaei A, Salarisedigh S, Sardari F, Khalili P, Eslami H. Investigation of the Bacterial Contamination of Dental Unit Waterlines and the Effectiveness of Flushing on the Contamination Level in Rafsanjan, Southeastern Iran. J Environ Health Sustain Dev 2025; 10 (3) :2771-2780
URL: http://jehsd.ssu.ac.ir/article-1-1009-en.html
URL: http://jehsd.ssu.ac.ir/article-1-1009-en.html
Occupational Environment Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran & Department of Environmental Health Engineering, School of Health, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
Full-Text [PDF 726 kb]
(118 Downloads)
| Abstract (HTML) (251 Views)
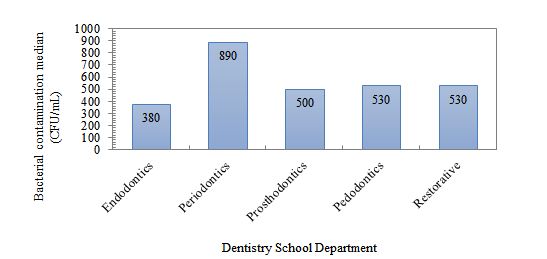
Figure 1: Bacterial contamination load of dental units in different departments of the dentistry school.
The median bacterial contamination (CFU/mL) in different parts of the unit, divided into different departments of the School of Dentistry, is presented in Table 1. Accordingly, the highest level of contamination was related to the scaler in the perioperative department, and the lowest level of contamination was related to the turbine in the pediatric department. Based on the statistical results of the Kruskal-Wallis test, there was no significant difference between the levels of contamination in different parts of the unit, divided into different departments (P ˃ 0.05)
Table 1: Median and interquartile range of heterotrophic bacteria in different parts of the
units in dentistry departments (n= 120)
Table 2: Percentage prevalence of identified bacterial types in different departments of the School of Dentistry (n=120)
Table 3: Heterotrophic bacteria prevalence in DUWLs according to flushing procedure
Table 4: Contamination levels of DUWLs from Dentistry school at different times according to ADA standards
Table 5: Comparison of contamination levels of unit water samples in different sections according to ADA standards
Discussion
Dental Unit Water Contamination Rate
This study showed that the contamination level in DUWLs at the Rafsanjan dental school was above the permissible limit set by the American Dental Association (500 CFU/mL) in 53.2% of the samples. These findings are consistent with the results of similar studies in Iran and other countries, especially in areas where DUWLs are not properly maintained and disinfected 1, 11. In the study by Buitrago et al., the rate of dental unit water contamination was reported to be 21% 21, which was lower than that in the present study. Yazdanbakhsh et al. reportedae bacterial contamination rate of Shahrood dental school to be 64% 17, which was higher than that in our study. Ghaem Maghami et al., reported the water contamination of Shahid Beheshti dental school to be 50% 18. Studies have shown that the duration of use of the dental units (years of use) increases the thickness of the biofilm layer and, as a result, increases the level of contamination 22. Water contamination in dental units can be due to two main reasons: the microbial flora of the patients' mouths, which can enter the unit's water supply system due to the suction effect and return of the patient's saliva (backflow) through suction or the turbine head duct, and the stable microbial environment deposited in the unit's water pipes, or the biofilm, which acts as a potential source of contamination 23.
The results of this study showed that the highest level of contamination was observed in the periodontal department. In the study by Blaszczyk et al., the highest level of contamination was in the perioral section, which was consistent with our study 24. In the study by Hajisadeghi et al., the lowest level of contamination was in the perioral section, which was inconsistent with our study and could be related to the level of operation of the units or the condition of the municipal water piping 25. According to previous studies, the longer the dental unit is inactive and the more water remains in the unit lines or pipes, the higher the contamination level of the outlet water. Therefore, it can be said that in our study, the reason for the higher contamination level in the periodontal department could be related to the less active units and less use of power in this department, as well as the use of manual scalers by lower-entry students and the unused scalers during that period of time 26, 27. In this study, the lowest contamination level was recorded in the endodontics department, which may be due to the continuous use of units and better adherence to the hygiene protocols.
The results of this study showed that the highest contamination rate was related to the scaler part, and the lowest contamination rate was related to the turbine part of the units. In the study by Abbasi et al., the highest contamination rate was related to the scaler, which was consistent with our study 23. In the study by Aghakochekzadeh et al., the highest contamination rate was related to the turbine, which was inconsistent with our study and could be related to a different statistical population 28.
Bacterial species
The most common bacterial species grown in our study were gram-positive bacilli (diphtheroid, spore-forming), Micrococcus, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Streptococcus, and coliforms. In the study by Abbasi et al., the most commonly reported species were gram-positive bacilli, which is consistent with our results 23. In the study by Aghakochekzadeh et al., the species found in order of prevalence were Escherichia coli, Klebsiella, non-pathogenic Staphylococcus, and Micrococcus 28. Moradania et al. reported the presence of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and coliforms in the unit water 29. In our study, Escherichia coli was not detected in any of the samples, although species in the coliform group were detected, all of which were non-intestinal or non-fecal. These species can also be commonly identified on surfaces, skin, and saliva 29. In addition, the presence of microorganisms such as Staphylococcus epidermidis can indicate contamination due to the return of patient saliva through suction or the turbine head duct into the unit water duct 30.
In addition, identification of bacterial species indicated the presence of potentially pathogenic microorganisms such as Staphylococcus aureus, Pseudomonas aeruginosa, and coliforms, which can pose a high health risk to patients with weakened immune systems 26.
The effect of flushing
A key finding of this study was the significant reduction in water contamination after a 30-second flush. This result is consistent with the recommendations of the ADA and the Centers for Disease Control (CDC) 31. Although flushing cannot completely remove biofilms, it plays an important role in reducing the microbial load as a simple, low-cost, and immediate method of intervention. The results of this study also showed that there was no statistically significant difference between samples before and after daily work; this indicates that daily activities alone do not increase contamination, but that there is already a baseline contamination 32.
This study showed that flushing at the beginning of the day and before starting work is significantly effective in reducing water contamination levels. This emphasizes the implementation of the American Dental Association guidelines, which require flushing before starting the daily work of the unit, between two patients, and after completing the daily work. The studies by Hosseini Mehraban et al., 30, Aghakouchakzadeh et al., 28, Hajisadeghi et al., 25, and Khondian et al. 33 are also consistent with the present study.
In the present study, 77.5% of the initial samples (before flushing) had contamination higher than the ADA standard, and this contamination decreased to 30% in the samples taken after flushing. According to the results obtained in this and other existing studies, flushing seems to be the best and most practical method for contamination control. However, it should be noted that this is not a perfect method because it cannot remove biofilms attached to the walls of the water path, which requires more detailed and complete studies in this field. In this study, bacterial counts in samples taken after work showed that the level of contamination decreased compared to samples taken before work. The high level of contamination before work could be due to the stagnation of water in the unit pipes at the beginning of the working day, and the reason for the decrease in microbial load after work could be due to the unit being active and the water being circulated 34. In the study by Abbasi et al., the level of contamination in samples collected at the beginning of the workday was higher than that in samples taken after work 23, which is consistent with the present study.
According to the ADA guidelines for controlling dental unit water contamination, and the Centers for Disease Control and Prevention (CDC), the bacterial concentration of dental unit water used in nonsurgical procedures should be less than or equal to 500 CFU/mL 31. Sterile saline or sterile water should be used as a coolant and rinsed during surgical procedures 21. The ADA recommends using stored water that is not connected to city water, cleaning air and water outlets daily, using chemical compounds to remove microbes from water, using special filters to control dental unit water, and flushing for 30 s before starting work to control and limit contamination 31. In a study conducted by Pankhurst et al., the results showed that installing a valve that prevents fluid from flowing back from the patient's mouth into the unit's water system reduces contamination 35. In a study by Berlutti et al. on the effect of an anti-retraction device on preventing microbial contamination of dental unit water lines, they concluded that even installing an anti-retraction device did not prevent fluid from flowing back from the patient's mouth into the unit's water system in 74% of cases when the turbine stopped moving, resulting in cross-infection between patients 36.
Conclusion
In this study, the bacterial contamination of the DUWLs in Rafsanjan Dental School exceeded the acceptable standard in 53.2% of the samples. The presence of pathogenic microorganisms, such as Staphylococcus aureus, Pseudomonas aeruginosa, and coliforms, indicates a potential risk of infection transmission in the dental environment. In the present study, the results before and after the flushing procedure showed a significant reduction in the microbial load in all devices, which is also recommended by the ADA as one of the obvious factors in reducing contamination before starting work. Therefore, flushing before work is recommended as a reliable and accessible method for all departments to reduce the microbial load. In addition, flushing alone cannot completely remove biofilms located in pipes. As a result, the use of complementary methods such as the use of non-return valves, periodic disinfection with chemicals, and the installation of continuous disinfection systems is recommended
Acknowledgements
This study is the result of a research thesis by a dentistry student at Rafsanjan University of Medical Sciences with code 4030027 and an ethical approval code IR.RUMS.REC.1403.087. We would like to thank Rafsanjan University of Medical Sciences for its financial and moral support of this study.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
Rafsanjan University of Medical Sciences, Rafsanjan, Iran
Ethical Considerations
This study approved by Rafsanjan University of Medical Sciences with code 4030027 and ethical code IR.RUMS.REC.1403.087.
Code of Ethics
IR.RUMS.REC.1403.087.
Authors' Contributions
All authors contributed to the study conception and design. Data collection were performed by Amirreza Isaei, and data analysis were performed by Somayeh Salari-Sedigh, Farimah Sardari, Hadi Eslami and Parvin Khalili. The first draft written and revision of the manuscript were performed by Amirreza Isaei and Hadi Eslami. All authors read and approved the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Bayani M, Raisolvaezin K, Almasi-Hashiani A, et al. Bacterial biofilm prevalence in dental unit waterlines: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):158.
2. Barbot V, Robert A, Rodier M-H, et al. Update on infectious risks associated with dental unit waterlines. FEMS Immunol Med Microbiol. 2012;65(2):196-204.
3. Zhang K, Li X, Yu C, et al. Promising therapeutic strategies against microbial biofilm challenges. Front Cell Infect Microbiol. 2020;10:359.
4. Spagnolo AM, Sartini M, Cristina ML. Microbial contamination of dental unit waterlines and potential risk of infection: a narrative review. Pathogens. 2020;9(8):651.
5. Veena H, Mahantesha S, Joseph PA, et al. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. 2015;8(3):260-5.
6. Watanabe A, Tamaki N, Yokota K, et al. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J Hosp Infect. 2018;99(3):303-5.
7. Kader CB, de Smidt O, Oosthuysen J. Water Quality and Biofilm Formation in Dental Unit Waterline Systems in Mangaung, South Africa. International Dental Journal. 2025.
8. Salmanov AG, Bondar TP, Lykhota KM, et al. Microbial contamination of dental unit waterlines systems in Ukraine: results a multicenter study (2020-2022). Wiadomosci lekarskie (Warsaw, Poland: 1960). 2025;78(6):974-81.
9. Samaranayake L, Fakhruddin K, Sobon N, et al. Dental unit waterlines: disinfection and management. Int Dent J. 2024;74:S437-S45.
10. Khajezadeh M, Mohseni F, Khaledi A, et al. Contamination of dental unit water lines (DUWL) with Legionella pneumophila and Pseudomonas aeruginosa; A Middle East systematic review and meta-analysis. Eur J Microbiol Immunol (Bp). 2023;12(4):93-9.
11. Zemouri C, de Soet H, Crielaard W, et al. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12(5):e0178007.
12. Baudet A, Lizon J, Lozniewski A, et al. Bacterial contamination of new dental unit waterlines and efficacy of shock disinfection. BMC Microbiol. 2024;24(1):529.
13. Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, et al. Pediatric dental clinic–associated outbreak of Mycobacterium abscessus infection. J Pediatric Infect Dis Soc. 2017;6(3):e116-e22.
14. Singh J, O’Donnell K, Nieves DJ, et al., editors. Invasive Mycobacterium abscessus outbreak at a pediatric dental clinic. Open Forum Infectious Diseases; 2021: Oxford University Press US; 8(6):165.
15. Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. The Lancet. 2012;379(9816):684.
16. Schönning C, Jernberg C, Klingenberg D, et al. Legionellosis acquired through a dental unit: a case study. J Hosp Infect. 2017;96(1):89-92.
17. Yazdanbakhsh A, Roudbari A, Nazemi S, et al. Evaluation of bacterial contamination of water supply in dental unit water lines at Shahroud dental offices 2015. Journal of Knowledge & Health in Basic Medical Sciences. 2016;1(1):49-54.
18. Ghaem Maghami A, Mahdipour M, Goudarzi H. The rate of bacterial contamination in dental units water supply at shahid behashti dental school–1999. Journal of Dental School. 2003;21(1):103-9.
19. STANDARD B, ISO. Water quality—Sampling for microbiological analysis. BRITISH: the International Organization for Standardization; 2006.
20. Köster W, Egli T, Ashbolt N, et al. Analytical methods for microbiological water quality testing. Assessing microbial safety of drinking water. 2003:237.
21. Buitrago JM, Kolbe RJ, Siqueira MF. Dental unit waterline testing practices: an 11-Year retrospective study. BMC Oral Health. 2023;23(1):867.
22. Kgabi SP, Mthethwa SR. The effect of A-dec ICX TM on microbiological water quality in self-contained dental units' water systems. South African Dental Journal. 2020;75(7):367-72.
23. Abbasi F, Saderi H, Rezaei Bonab M, et al. Survey of bacterial contamination rate of water in dental units of School of Dentistry, Shahed University. Daneshvar Medicine. 2020;25(5):15-20.
24. Błaszczyk B, Pajączkowska M, Nowicka J, et al. Microbiological evaluation of water used in dental units. Water. 2022;14(6):915.
25. Hajisadeghi S, Aghaali M, Aghighi Hatamipour S, et al. Evaluation of bacterial contamination level of waterlines in Dental Units in Dentistry School of Qom University of Medical Sciences in 2018. Qom University of Medical Sciences Journal. 2020;14(8):30-8.
26. Hoogenkamp MA, Brandt BW, de Soet JJ, et al. An in-vitro dynamic flow model for translational research into dental unit water system biofilms. J Microbiol Methods. 2020;171:105879.
27. Shajahan IF, Kandaswamy D, Lakshminarayanan L, et al. Substantivity of hypochlorous acid-based disinfectant against biofilm formation in the dental unit waterlines. J Conserv Dent Endod. 2017;20(1):2-5.
28. Aghakouchakzadeh A, Mohamadzadeh M, Hamian M, et al. Evaluating bacterial contamination of water supply in Dental Units at Alborz University of Medical Science using microbial culture and colony count in 2019. Alborz University Medical Journal. 2020;9(4): 31-8.
29. Moradnia M, Noorisepehr M, Ghaderi S, et al. Investigation of microbial contamination in surfaces and waterlines of dental units in terms of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Coliforms. Journal of Environmental Health Engineering. 2021;8(2):185–95.
30. Hoseini mehrian Z, Naghmachi M, Zirakfard F, et al. A study on microbial quality of water used in the dentistry units. Armaghane-danesh. 2014;19(8):707-16.
31. American Dental Association. ADA statement on dental unit waterlines. The Journal of the American Dental Association. 1996;127(2):185-6.
32. Santiago JI, Huntington M, Johnston A, et al. Microbial contamination of dental unit waterlines: short-and long-term effects of flushing. Gen Dent. 1994;42(6):528-35.
33. Khorakian F, Movahhed T, Karbasi S, et al. Frequency evaluation of Pseudomonas SPP contamination in the dental unit water line system at Mashhad dental school. Res Dent Sci. 2019;16(3):209-1.
34. Smith A. The devil is in the validation and design; managing the risk from opportunistic pathogens in the dental unit. J Hosp Infect. 2021;114:61-2.
35. Pankhurst CL, Johnson N, Woods R. Microbial contamination of dental unit waterlines: the scientific argument. Int Dent J. 1998;48(4):359-68.
36. Berlutti F, Testarelli L, Vaia F, et al. Efficacy of anti-retraction devices in preventing bacterial contamination of dental unit water lines. Journal of Dentistry. 2003;31(2):105-10.
Full-Text: (90 Views)
Investigation of the Bacterial Contamination of Dental Unit Waterlines and the Effectiveness of Flushing on the Contamination Level in Rafsanjan, Southeastern Iran
Amirreza Isaei 1, Somayeh Salarisedigh 2, Farimah Sardari 3, Parvin Khalili 4, Hadi Eslami 5,6*
1 Student Research Committee, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
2, Department of Periodontics, School of Dentistry, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3 Department of Oral Medicine, School of Dentistry, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
4 Department of Epidemiology, School of Health, Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
5 Occupational Environment Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
6 Department of Environmental Health Engineering, School of Health, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
Amirreza Isaei 1, Somayeh Salarisedigh 2, Farimah Sardari 3, Parvin Khalili 4, Hadi Eslami 5,6*
1 Student Research Committee, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
2, Department of Periodontics, School of Dentistry, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3 Department of Oral Medicine, School of Dentistry, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
4 Department of Epidemiology, School of Health, Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
5 Occupational Environment Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
6 Department of Environmental Health Engineering, School of Health, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Dental unit water lines (DUWLs) are potential sources of microbial contamination that threaten patients and dental personnel. This study aimed to determine the bacterial quantity and quality of DUWLs in Dental School in Rafsanjan and to determine the effect of flushing on the contamination rate. Materials and Methods: In this cross-sectional study, 124 water samples were collected from 20 active units in five departments (endodontics, periodontics, pediatrics, prosthetics, and restorations) at Rafsanjan Dental School (July 2024). Sampling was carried out using a standard method from the water inlet and the connection point of the turbine and scaler in three stages: before work, after 30-second flushing, and after work. To identify the bacterial contamination load, heterotrophic plate counting (HPC), Gram staining, and standard biochemical tests for each bacterial species were used. Kruskal-Wallis and Mann-Whitney tests were used to analyze the data. Results: The results showed that the contamination level in 53.2% of the samples was above the permissible limit. The highest contamination level was observed in the periodontics department (890 CFU/mL), and the lowest was in the endodontics department (380 CFU/mL). 30-second flushing significantly reduced contamination and the number of bacteria (p ≤ 0.001), while the difference between contamination levels in different departments and components of the dental units was not significant (p ˃ 0.05). Conclusion: Given the contamination of DUWLs, continuous water disinfection, cleaning of water lines, and flushing before and during work in dental units are recommended. |
|
Article History: Received: 13 May 2025 Accepted: 20 July 2025 |
||
*Corresponding Author: Hadi Eslami Email: hadieslami1986@yahoo.com Tel: +98 917 7094695 |
||
Keywords: Aerosols; Dental Unit Waterlines; Dentistry; Bacterial Contamination; Water Pollution. |
Citation: Isaei A, Salarisedigh S, Sardari F, et al. Investigation of the Bacterial Contamination of Dental Unit Waterlines and the Effectiveness of Flushing on the Contamination Level in Rafsanjan, Southeastern Iran. J Environ Health Sustain Dev. 2025; 10(3): 2771-80.
Introduction
Dental unit waterlines (DUWLs) are essential components of dental treatment systems that supply the water required to operate several dental instruments and devices, including turbines, air and water pumps, and ultrasonic scalers 1. The structure of DUWLs leads to the rapid formation and growth of biofilms 2. The average water temperature in DUWLs is in the range of 20 to 30 °C, which is suitable for the growth and formation of biofilms 2. However, the long-term residence of water in DUWLs causes the formation of biofilms, and the contamination of the water outlet from the dental unit originates from the biofilm attached to the walls of its pipes 3. In addition, contamination of DUWLs can be caused by saliva reflux from the patient's oral cavity, which occurs when the equipment creates negative pressure 4.
Contact of patients and staff with contaminated water or aerosols through the digestive tract and the entry of aerosols into the respiratory system through the air can lead to infection, especially in immunocompromised individuals, children, and the elderly 5, 6. Studies have reported the prevalence of various microbial contaminants, including bacteria, fungi, and protozoa, in DUWLs 1, 3. However, the most common contaminations are related to various bacterial species. Studies have reported the presence of heterotrophic pathogenic bacteria, such as Staphylococcus aureus, Legionella, Pseudomonas, especially Pseudomonas aeruginosa, and Escherichia coli in DUWL systems; in many cases, the concentrations of these contaminants were higher than the permissible limit set by the American Dental Association (ADA), which is 500 colony-forming units per milliliter (CFU/mL) 7-9.
High concentrations of Pseudomonas aeruginosa have been implicated in pulmonary infections in patients with cystic fibrosis, and a dentist reportedly died of pneumonia after exposure to contaminated dental unit water 10. Indeed, bacteria transmitted through aerosols can cause illnesses such as influenza and the common cold, as well as respiratory diseases such as tuberculosis and Legionnaires' disease 6, 11. Studies have shown that Infections caused by Mycobacterium abscessus in 95 children (24 and 71 children) in two pediatric dental clinics in the United States were directly linked to contaminated water from dental units used for pulpotomy 12-14. Also, two deaths in elderly patients, one in Italy and the other in Sweden, have been reported after exposure to water contaminated with L. pneumophila in DUWLs 12, 15, 16. Studies conducted in Iran have also reported varying degrees of bacterial contamination in dental units. In the study by Yazdanbakhsh et al., the bacterial contamination rate of water in Shahrood dental units was reported to be 64% 17, and in the study by Ghaem Maghami et al., the bacterial contamination rate of water in Shahid Beheshti dental units was reported to be 50% 18.
Epidemiological research in different countries and cities has yielded different results, and it seems necessary to conduct extensive and comprehensive research in this field to determine the causes and factors of contamination and methods of their control and elimination. Therefore, considering that water contamination in dental units can pose risks to the health of patients, personnel, and dentists, and considering the necessity of conducting these studies in all parts of the world with the aim of updating information in this field in order to determine the causes and factors of contamination and methods of their control and elimination, and also with the aim of providing better and more effective treatments, this study was conducted to evaluate the level of bacterial contamination of the DUWLs at the Rafsanjan Dental School and determine the effectiveness of the flushing method in reducing microbial contamination.
Materials and Methods
Sampling
This descriptive, cross-sectional study was conducted in July 2024 at the School of Dentistry, Rafsanjan University of Medical Sciences. A total of 124 water samples were collected from 20 active units in five departments: endodontics, periodontics, pediatrics, prosthetics, and restorative dentistry. Samples were collected from three areas of each unit: the air and water pump, turbine connection, and ultrasonic scaler. Sampling was performed in three-time steps: before starting work, after finishing work, and after performing a 30-second flushing. Four municipal water samples were collected for the control group. Sampling was carried out according to the standard for microbial water sampling, and samples were collected aseptically in sterile containers and immediately transferred to the microbiology laboratory 19. All samples were transported to the laboratory and tested within two hours of collection.
Microbial Analysis
Heterotrophic plate counting (HPC) or standard colony counting was used to determine the number of bacterial colonies. Samples were plated on nutrient agar using the pour plate method and incubated for 48 h at 35–37°C. The results are reported as CFU/mL. To identify the bacterial species, the colonies were subjected to Gram staining and biochemical tests, including IMViC, catalase, coagulase, DNase, and oxidase. A catalase test was performed to detect colonies suspected to be gram-positive cocci. The catalase-positive and catalase-negative bacteria were staphylococci and streptococci, respectively. In catalase-positive cases, deoxyribonuclease (DNase) and coagulase tests were used to diagnose Staphylococcus aureus. The Novobiocin test was used to distinguish Staphylococcus epidermidis from Staphylococcus saprophyticus. In the case of gram-negative bacilli, the oxidase test is used to differentiate Pseudomonas from Enterobacteriaceae, which shows a green pigment. The IMViC test was also used to differentiate between different bacteria in the Enterobacteriaceae family. The IMViC test is a set of four different biochemical tests, including the indole, Methyl Red, Voges-Proskauer, and Citrate Utilization tests, which are used to identify and differentiate bacteria, especially members of the Enterobacteriaceae family, and the samples were tested according to the standard method 20.
Statistical analysis
Data were analyzed using SPSS version 22. Descriptive statistics, including median, interquartile range, and frequency, were calculated. Mann-Whitney U and Kruskal-Wallis tests were used to compare the groups. The level of statistical significance was set at ˂ 0.05.
Results
Bacterial concentration in DUWLs
The median bacterial contamination (CFU/mL) in the different departments is shown in Figure 1. The highest contamination was observed in the periodontics department (890 CFU/mL), and the lowest was in the endodontics department (380 CFU/mL). The median values were for the prosthetic, restorative (530 CFU/mL), and pediatric (500 CFU/mL) departments. However, the Kruskal-Wallis test did not show a significant difference between the contamination of the departments (p = 0.736)
Dental unit waterlines (DUWLs) are essential components of dental treatment systems that supply the water required to operate several dental instruments and devices, including turbines, air and water pumps, and ultrasonic scalers 1. The structure of DUWLs leads to the rapid formation and growth of biofilms 2. The average water temperature in DUWLs is in the range of 20 to 30 °C, which is suitable for the growth and formation of biofilms 2. However, the long-term residence of water in DUWLs causes the formation of biofilms, and the contamination of the water outlet from the dental unit originates from the biofilm attached to the walls of its pipes 3. In addition, contamination of DUWLs can be caused by saliva reflux from the patient's oral cavity, which occurs when the equipment creates negative pressure 4.
Contact of patients and staff with contaminated water or aerosols through the digestive tract and the entry of aerosols into the respiratory system through the air can lead to infection, especially in immunocompromised individuals, children, and the elderly 5, 6. Studies have reported the prevalence of various microbial contaminants, including bacteria, fungi, and protozoa, in DUWLs 1, 3. However, the most common contaminations are related to various bacterial species. Studies have reported the presence of heterotrophic pathogenic bacteria, such as Staphylococcus aureus, Legionella, Pseudomonas, especially Pseudomonas aeruginosa, and Escherichia coli in DUWL systems; in many cases, the concentrations of these contaminants were higher than the permissible limit set by the American Dental Association (ADA), which is 500 colony-forming units per milliliter (CFU/mL) 7-9.
High concentrations of Pseudomonas aeruginosa have been implicated in pulmonary infections in patients with cystic fibrosis, and a dentist reportedly died of pneumonia after exposure to contaminated dental unit water 10. Indeed, bacteria transmitted through aerosols can cause illnesses such as influenza and the common cold, as well as respiratory diseases such as tuberculosis and Legionnaires' disease 6, 11. Studies have shown that Infections caused by Mycobacterium abscessus in 95 children (24 and 71 children) in two pediatric dental clinics in the United States were directly linked to contaminated water from dental units used for pulpotomy 12-14. Also, two deaths in elderly patients, one in Italy and the other in Sweden, have been reported after exposure to water contaminated with L. pneumophila in DUWLs 12, 15, 16. Studies conducted in Iran have also reported varying degrees of bacterial contamination in dental units. In the study by Yazdanbakhsh et al., the bacterial contamination rate of water in Shahrood dental units was reported to be 64% 17, and in the study by Ghaem Maghami et al., the bacterial contamination rate of water in Shahid Beheshti dental units was reported to be 50% 18.
Epidemiological research in different countries and cities has yielded different results, and it seems necessary to conduct extensive and comprehensive research in this field to determine the causes and factors of contamination and methods of their control and elimination. Therefore, considering that water contamination in dental units can pose risks to the health of patients, personnel, and dentists, and considering the necessity of conducting these studies in all parts of the world with the aim of updating information in this field in order to determine the causes and factors of contamination and methods of their control and elimination, and also with the aim of providing better and more effective treatments, this study was conducted to evaluate the level of bacterial contamination of the DUWLs at the Rafsanjan Dental School and determine the effectiveness of the flushing method in reducing microbial contamination.
Materials and Methods
Sampling
This descriptive, cross-sectional study was conducted in July 2024 at the School of Dentistry, Rafsanjan University of Medical Sciences. A total of 124 water samples were collected from 20 active units in five departments: endodontics, periodontics, pediatrics, prosthetics, and restorative dentistry. Samples were collected from three areas of each unit: the air and water pump, turbine connection, and ultrasonic scaler. Sampling was performed in three-time steps: before starting work, after finishing work, and after performing a 30-second flushing. Four municipal water samples were collected for the control group. Sampling was carried out according to the standard for microbial water sampling, and samples were collected aseptically in sterile containers and immediately transferred to the microbiology laboratory 19. All samples were transported to the laboratory and tested within two hours of collection.
Microbial Analysis
Heterotrophic plate counting (HPC) or standard colony counting was used to determine the number of bacterial colonies. Samples were plated on nutrient agar using the pour plate method and incubated for 48 h at 35–37°C. The results are reported as CFU/mL. To identify the bacterial species, the colonies were subjected to Gram staining and biochemical tests, including IMViC, catalase, coagulase, DNase, and oxidase. A catalase test was performed to detect colonies suspected to be gram-positive cocci. The catalase-positive and catalase-negative bacteria were staphylococci and streptococci, respectively. In catalase-positive cases, deoxyribonuclease (DNase) and coagulase tests were used to diagnose Staphylococcus aureus. The Novobiocin test was used to distinguish Staphylococcus epidermidis from Staphylococcus saprophyticus. In the case of gram-negative bacilli, the oxidase test is used to differentiate Pseudomonas from Enterobacteriaceae, which shows a green pigment. The IMViC test was also used to differentiate between different bacteria in the Enterobacteriaceae family. The IMViC test is a set of four different biochemical tests, including the indole, Methyl Red, Voges-Proskauer, and Citrate Utilization tests, which are used to identify and differentiate bacteria, especially members of the Enterobacteriaceae family, and the samples were tested according to the standard method 20.
Statistical analysis
Data were analyzed using SPSS version 22. Descriptive statistics, including median, interquartile range, and frequency, were calculated. Mann-Whitney U and Kruskal-Wallis tests were used to compare the groups. The level of statistical significance was set at ˂ 0.05.
Results
Bacterial concentration in DUWLs
The median bacterial contamination (CFU/mL) in the different departments is shown in Figure 1. The highest contamination was observed in the periodontics department (890 CFU/mL), and the lowest was in the endodontics department (380 CFU/mL). The median values were for the prosthetic, restorative (530 CFU/mL), and pediatric (500 CFU/mL) departments. However, the Kruskal-Wallis test did not show a significant difference between the contamination of the departments (p = 0.736)

Figure 1: Bacterial contamination load of dental units in different departments of the dentistry school.
The median bacterial contamination (CFU/mL) in different parts of the unit, divided into different departments of the School of Dentistry, is presented in Table 1. Accordingly, the highest level of contamination was related to the scaler in the perioperative department, and the lowest level of contamination was related to the turbine in the pediatric department. Based on the statistical results of the Kruskal-Wallis test, there was no significant difference between the levels of contamination in different parts of the unit, divided into different departments (P ˃ 0.05)
Table 1: Median and interquartile range of heterotrophic bacteria in different parts of the
units in dentistry departments (n= 120)
| Dentistry departments | Units | Median (CFU/mL) | Interquartile range (CFU/mL) | P-value |
| Endodontics | Air-water syringe | 430 | 320-1952.5 | 0.707 |
| Air turbine handpiece | 510 | 65.5-1975 | ||
| Periodontics | Air-water syringe | 780 | 290-1657.5 | 0.285 |
| Air turbine handpiece | 995 | 383.75-3950 | ||
| Pedodontics | Air-water syringe | 895 | 130-3049 | 0.401 |
| Air turbine handpiece | 400 | 222.5-1882.5 | ||
| Prosthodontics | Air-water syringe | 740 | 382.5-1050 | 0.885 |
| Air turbine handpiece | 527.5 | 410-3982.5 | ||
| Restorative | Air-water syringe | 417.5 | 335-830 | 0.236 |
| Air turbine handpiece | 895 | 382.5-985 |
Microbial species
Microscopic and biochemical analyses revealed the presence of various bacterial species. The prevalence of various microorganisms in the collected samples and the prevalence of each microorganism in different sections are presented in Table 2. The highest prevalence was related to gram-positive bacilli (69.2%), and the lowest prevalence was related to Streptococcus (5.8%) and coliforms (5.8%).
Microscopic and biochemical analyses revealed the presence of various bacterial species. The prevalence of various microorganisms in the collected samples and the prevalence of each microorganism in different sections are presented in Table 2. The highest prevalence was related to gram-positive bacilli (69.2%), and the lowest prevalence was related to Streptococcus (5.8%) and coliforms (5.8%).
Table 2: Percentage prevalence of identified bacterial types in different departments of the School of Dentistry (n=120)
| Microorganisms | Number of Contaminated Samples (%) |
Faculty departments | P-value |
||||
| Restorative | Prosthodontics | Pedodontics | Periodontics | Endodontics | |||
| Gram-positive bacillus | 83(69.2) | 22.9% | 22.9% | 19.3% | 14.5% | 20.5% | 0.166 |
| Micrococcus | 32(26.7) | 12.5% | 18.8% | 21.9% | 25% | 21.9% | 0.743 |
| Staph aureus | 23(19.2) | 26.1% | 34.8% | 13% | 13% | 13% | 0.223 |
| Staph epidermidis | 17(14.2) | 23.5% | 23.5% | 11.8% | 11.8% | 29.4% | 0.651 |
| Pseudomonas aeruginosa | 12(10) | 33.3% | 33.3% | 0% | 16.7% | 16.7% | 0.269 |
| Streptococcus | 7(5.8) | 28.6% | 14.3% | 42.9% | 14.3% | 0% | 0.414 |
| Coliforms | 7(5.8) | 28.6% | 14.3% | 42.9% | 14.3% | 0% | 0.414 |
Effect of Flushing
The median bacterial contamination (CFU/mL) by the flushing procedure is presented in Table 3. The contamination level decreased after flushing, and according to the Mann-Whitney test, there was a significant difference between the contamination levels of the samples before and after flushing (p ≤ 0.001). After 30 s of flushing, a significant decrease in contamination was observed; the median CFU decreased from 905 to 350, which was statistically significant (p ≤ 0.001). In contrast, the difference between the samples before and after daily clinical work was not significant (p = 0.152). The contamination level of the samples after work was lower than that of the samples before work, but based on the statistical results of the Mann-Whitney test, there was no significant difference between the contamination level of the samples before and after work (p=0.152)
The median bacterial contamination (CFU/mL) by the flushing procedure is presented in Table 3. The contamination level decreased after flushing, and according to the Mann-Whitney test, there was a significant difference between the contamination levels of the samples before and after flushing (p ≤ 0.001). After 30 s of flushing, a significant decrease in contamination was observed; the median CFU decreased from 905 to 350, which was statistically significant (p ≤ 0.001). In contrast, the difference between the samples before and after daily clinical work was not significant (p = 0.152). The contamination level of the samples after work was lower than that of the samples before work, but based on the statistical results of the Mann-Whitney test, there was no significant difference between the contamination level of the samples before and after work (p=0.152)
Table 3: Heterotrophic bacteria prevalence in DUWLs according to flushing procedure
| Sampling time | Number of samples | Median (CFU/mL) | Interquartile range (CFU/mL) | p-value |
| Before work | 40 | 905 | 612.5-2300 | - |
| After 30 seconds of flushing | 40 | 350 | 6-530 | < 0.001 |
| After work | 40 | 685 | 395-2000 | 0.152 |
Table 4 shows how many samples had contamination higher than the ADA standard and how many had contamination lower than the ADA standard at different sampling times. Based on the results, 77.5% of the initial samples and those before flushing had contamination levels higher than the ADA standard, which decreased to 30% in the samples taken after flushing. In addition, 57.5% of the samples taken after work had contamination levels higher than the standard. Based on the statistical results of the Chi-Square test at different sampling times, there was a significant difference between the number of samples with contamination higher than the ADA standard and those with contamination lower than the ADA standard (p ≤ 0.001).
Table 4: Contamination levels of DUWLs from Dentistry school at different times according to ADA standards
| Sampling time | > 500 CFU/mL | ≤ 500 CFU/mL | p-value | |
| Before work | Number | 31 | 9 | < 0.001 |
| Percent | 77.5% | 22.5% | ||
| After 30 seconds of flushing | Number | 12 | 28 | |
| Percent | 30% | 70% | ||
| After work | Number | 23 | 17 | |
| Percent | 57.5% | 42.5% | ||
Table 5 compares the contamination levels of unit water samples in different departments of the dental school according to ADA standards. The results showed that overall, 53.2% of the samples had contamination levels above the permissible limit (500 CFU/mL), most of which were related to the periodontics and prosthetics departments.
Table 5: Comparison of contamination levels of unit water samples in different sections according to ADA standards
| Dentistry departments | > 500 cfu/mL | < = 500 cfu/mL | p-value | |
| Endodontics | Number | 11 | 14 | 0.634 |
| Percent | 44% | 56% | ||
| Periodontics | Number | 16 | 9 | |
| Percent | 64% | 36% | ||
| Pedodontics | Number | 12 | 13 | |
| Percent | 48% | 52% | ||
| Prosthodontics | Number | 14 | 10 | |
| Percent | 58.3% | 41.7% | ||
| Restorative | Number | 13 | 12 | |
| Percent | 52% | 48% | ||
| Total | Number | 66 | 58 | |
| Percent | 53.2% | 48.8% | ||
Discussion
Dental Unit Water Contamination Rate
This study showed that the contamination level in DUWLs at the Rafsanjan dental school was above the permissible limit set by the American Dental Association (500 CFU/mL) in 53.2% of the samples. These findings are consistent with the results of similar studies in Iran and other countries, especially in areas where DUWLs are not properly maintained and disinfected 1, 11. In the study by Buitrago et al., the rate of dental unit water contamination was reported to be 21% 21, which was lower than that in the present study. Yazdanbakhsh et al. reportedae bacterial contamination rate of Shahrood dental school to be 64% 17, which was higher than that in our study. Ghaem Maghami et al., reported the water contamination of Shahid Beheshti dental school to be 50% 18. Studies have shown that the duration of use of the dental units (years of use) increases the thickness of the biofilm layer and, as a result, increases the level of contamination 22. Water contamination in dental units can be due to two main reasons: the microbial flora of the patients' mouths, which can enter the unit's water supply system due to the suction effect and return of the patient's saliva (backflow) through suction or the turbine head duct, and the stable microbial environment deposited in the unit's water pipes, or the biofilm, which acts as a potential source of contamination 23.
The results of this study showed that the highest level of contamination was observed in the periodontal department. In the study by Blaszczyk et al., the highest level of contamination was in the perioral section, which was consistent with our study 24. In the study by Hajisadeghi et al., the lowest level of contamination was in the perioral section, which was inconsistent with our study and could be related to the level of operation of the units or the condition of the municipal water piping 25. According to previous studies, the longer the dental unit is inactive and the more water remains in the unit lines or pipes, the higher the contamination level of the outlet water. Therefore, it can be said that in our study, the reason for the higher contamination level in the periodontal department could be related to the less active units and less use of power in this department, as well as the use of manual scalers by lower-entry students and the unused scalers during that period of time 26, 27. In this study, the lowest contamination level was recorded in the endodontics department, which may be due to the continuous use of units and better adherence to the hygiene protocols.
The results of this study showed that the highest contamination rate was related to the scaler part, and the lowest contamination rate was related to the turbine part of the units. In the study by Abbasi et al., the highest contamination rate was related to the scaler, which was consistent with our study 23. In the study by Aghakochekzadeh et al., the highest contamination rate was related to the turbine, which was inconsistent with our study and could be related to a different statistical population 28.
Bacterial species
The most common bacterial species grown in our study were gram-positive bacilli (diphtheroid, spore-forming), Micrococcus, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Streptococcus, and coliforms. In the study by Abbasi et al., the most commonly reported species were gram-positive bacilli, which is consistent with our results 23. In the study by Aghakochekzadeh et al., the species found in order of prevalence were Escherichia coli, Klebsiella, non-pathogenic Staphylococcus, and Micrococcus 28. Moradania et al. reported the presence of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and coliforms in the unit water 29. In our study, Escherichia coli was not detected in any of the samples, although species in the coliform group were detected, all of which were non-intestinal or non-fecal. These species can also be commonly identified on surfaces, skin, and saliva 29. In addition, the presence of microorganisms such as Staphylococcus epidermidis can indicate contamination due to the return of patient saliva through suction or the turbine head duct into the unit water duct 30.
In addition, identification of bacterial species indicated the presence of potentially pathogenic microorganisms such as Staphylococcus aureus, Pseudomonas aeruginosa, and coliforms, which can pose a high health risk to patients with weakened immune systems 26.
The effect of flushing
A key finding of this study was the significant reduction in water contamination after a 30-second flush. This result is consistent with the recommendations of the ADA and the Centers for Disease Control (CDC) 31. Although flushing cannot completely remove biofilms, it plays an important role in reducing the microbial load as a simple, low-cost, and immediate method of intervention. The results of this study also showed that there was no statistically significant difference between samples before and after daily work; this indicates that daily activities alone do not increase contamination, but that there is already a baseline contamination 32.
This study showed that flushing at the beginning of the day and before starting work is significantly effective in reducing water contamination levels. This emphasizes the implementation of the American Dental Association guidelines, which require flushing before starting the daily work of the unit, between two patients, and after completing the daily work. The studies by Hosseini Mehraban et al., 30, Aghakouchakzadeh et al., 28, Hajisadeghi et al., 25, and Khondian et al. 33 are also consistent with the present study.
In the present study, 77.5% of the initial samples (before flushing) had contamination higher than the ADA standard, and this contamination decreased to 30% in the samples taken after flushing. According to the results obtained in this and other existing studies, flushing seems to be the best and most practical method for contamination control. However, it should be noted that this is not a perfect method because it cannot remove biofilms attached to the walls of the water path, which requires more detailed and complete studies in this field. In this study, bacterial counts in samples taken after work showed that the level of contamination decreased compared to samples taken before work. The high level of contamination before work could be due to the stagnation of water in the unit pipes at the beginning of the working day, and the reason for the decrease in microbial load after work could be due to the unit being active and the water being circulated 34. In the study by Abbasi et al., the level of contamination in samples collected at the beginning of the workday was higher than that in samples taken after work 23, which is consistent with the present study.
According to the ADA guidelines for controlling dental unit water contamination, and the Centers for Disease Control and Prevention (CDC), the bacterial concentration of dental unit water used in nonsurgical procedures should be less than or equal to 500 CFU/mL 31. Sterile saline or sterile water should be used as a coolant and rinsed during surgical procedures 21. The ADA recommends using stored water that is not connected to city water, cleaning air and water outlets daily, using chemical compounds to remove microbes from water, using special filters to control dental unit water, and flushing for 30 s before starting work to control and limit contamination 31. In a study conducted by Pankhurst et al., the results showed that installing a valve that prevents fluid from flowing back from the patient's mouth into the unit's water system reduces contamination 35. In a study by Berlutti et al. on the effect of an anti-retraction device on preventing microbial contamination of dental unit water lines, they concluded that even installing an anti-retraction device did not prevent fluid from flowing back from the patient's mouth into the unit's water system in 74% of cases when the turbine stopped moving, resulting in cross-infection between patients 36.
Conclusion
In this study, the bacterial contamination of the DUWLs in Rafsanjan Dental School exceeded the acceptable standard in 53.2% of the samples. The presence of pathogenic microorganisms, such as Staphylococcus aureus, Pseudomonas aeruginosa, and coliforms, indicates a potential risk of infection transmission in the dental environment. In the present study, the results before and after the flushing procedure showed a significant reduction in the microbial load in all devices, which is also recommended by the ADA as one of the obvious factors in reducing contamination before starting work. Therefore, flushing before work is recommended as a reliable and accessible method for all departments to reduce the microbial load. In addition, flushing alone cannot completely remove biofilms located in pipes. As a result, the use of complementary methods such as the use of non-return valves, periodic disinfection with chemicals, and the installation of continuous disinfection systems is recommended
Acknowledgements
This study is the result of a research thesis by a dentistry student at Rafsanjan University of Medical Sciences with code 4030027 and an ethical approval code IR.RUMS.REC.1403.087. We would like to thank Rafsanjan University of Medical Sciences for its financial and moral support of this study.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
Rafsanjan University of Medical Sciences, Rafsanjan, Iran
Ethical Considerations
This study approved by Rafsanjan University of Medical Sciences with code 4030027 and ethical code IR.RUMS.REC.1403.087.
Code of Ethics
IR.RUMS.REC.1403.087.
Authors' Contributions
All authors contributed to the study conception and design. Data collection were performed by Amirreza Isaei, and data analysis were performed by Somayeh Salari-Sedigh, Farimah Sardari, Hadi Eslami and Parvin Khalili. The first draft written and revision of the manuscript were performed by Amirreza Isaei and Hadi Eslami. All authors read and approved the final manuscript.
This is an Open-Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work for commercial use.
References
1. Bayani M, Raisolvaezin K, Almasi-Hashiani A, et al. Bacterial biofilm prevalence in dental unit waterlines: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):158.
2. Barbot V, Robert A, Rodier M-H, et al. Update on infectious risks associated with dental unit waterlines. FEMS Immunol Med Microbiol. 2012;65(2):196-204.
3. Zhang K, Li X, Yu C, et al. Promising therapeutic strategies against microbial biofilm challenges. Front Cell Infect Microbiol. 2020;10:359.
4. Spagnolo AM, Sartini M, Cristina ML. Microbial contamination of dental unit waterlines and potential risk of infection: a narrative review. Pathogens. 2020;9(8):651.
5. Veena H, Mahantesha S, Joseph PA, et al. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. 2015;8(3):260-5.
6. Watanabe A, Tamaki N, Yokota K, et al. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J Hosp Infect. 2018;99(3):303-5.
7. Kader CB, de Smidt O, Oosthuysen J. Water Quality and Biofilm Formation in Dental Unit Waterline Systems in Mangaung, South Africa. International Dental Journal. 2025.
8. Salmanov AG, Bondar TP, Lykhota KM, et al. Microbial contamination of dental unit waterlines systems in Ukraine: results a multicenter study (2020-2022). Wiadomosci lekarskie (Warsaw, Poland: 1960). 2025;78(6):974-81.
9. Samaranayake L, Fakhruddin K, Sobon N, et al. Dental unit waterlines: disinfection and management. Int Dent J. 2024;74:S437-S45.
10. Khajezadeh M, Mohseni F, Khaledi A, et al. Contamination of dental unit water lines (DUWL) with Legionella pneumophila and Pseudomonas aeruginosa; A Middle East systematic review and meta-analysis. Eur J Microbiol Immunol (Bp). 2023;12(4):93-9.
11. Zemouri C, de Soet H, Crielaard W, et al. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12(5):e0178007.
12. Baudet A, Lizon J, Lozniewski A, et al. Bacterial contamination of new dental unit waterlines and efficacy of shock disinfection. BMC Microbiol. 2024;24(1):529.
13. Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, et al. Pediatric dental clinic–associated outbreak of Mycobacterium abscessus infection. J Pediatric Infect Dis Soc. 2017;6(3):e116-e22.
14. Singh J, O’Donnell K, Nieves DJ, et al., editors. Invasive Mycobacterium abscessus outbreak at a pediatric dental clinic. Open Forum Infectious Diseases; 2021: Oxford University Press US; 8(6):165.
15. Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. The Lancet. 2012;379(9816):684.
16. Schönning C, Jernberg C, Klingenberg D, et al. Legionellosis acquired through a dental unit: a case study. J Hosp Infect. 2017;96(1):89-92.
17. Yazdanbakhsh A, Roudbari A, Nazemi S, et al. Evaluation of bacterial contamination of water supply in dental unit water lines at Shahroud dental offices 2015. Journal of Knowledge & Health in Basic Medical Sciences. 2016;1(1):49-54.
18. Ghaem Maghami A, Mahdipour M, Goudarzi H. The rate of bacterial contamination in dental units water supply at shahid behashti dental school–1999. Journal of Dental School. 2003;21(1):103-9.
19. STANDARD B, ISO. Water quality—Sampling for microbiological analysis. BRITISH: the International Organization for Standardization; 2006.
20. Köster W, Egli T, Ashbolt N, et al. Analytical methods for microbiological water quality testing. Assessing microbial safety of drinking water. 2003:237.
21. Buitrago JM, Kolbe RJ, Siqueira MF. Dental unit waterline testing practices: an 11-Year retrospective study. BMC Oral Health. 2023;23(1):867.
22. Kgabi SP, Mthethwa SR. The effect of A-dec ICX TM on microbiological water quality in self-contained dental units' water systems. South African Dental Journal. 2020;75(7):367-72.
23. Abbasi F, Saderi H, Rezaei Bonab M, et al. Survey of bacterial contamination rate of water in dental units of School of Dentistry, Shahed University. Daneshvar Medicine. 2020;25(5):15-20.
24. Błaszczyk B, Pajączkowska M, Nowicka J, et al. Microbiological evaluation of water used in dental units. Water. 2022;14(6):915.
25. Hajisadeghi S, Aghaali M, Aghighi Hatamipour S, et al. Evaluation of bacterial contamination level of waterlines in Dental Units in Dentistry School of Qom University of Medical Sciences in 2018. Qom University of Medical Sciences Journal. 2020;14(8):30-8.
26. Hoogenkamp MA, Brandt BW, de Soet JJ, et al. An in-vitro dynamic flow model for translational research into dental unit water system biofilms. J Microbiol Methods. 2020;171:105879.
27. Shajahan IF, Kandaswamy D, Lakshminarayanan L, et al. Substantivity of hypochlorous acid-based disinfectant against biofilm formation in the dental unit waterlines. J Conserv Dent Endod. 2017;20(1):2-5.
28. Aghakouchakzadeh A, Mohamadzadeh M, Hamian M, et al. Evaluating bacterial contamination of water supply in Dental Units at Alborz University of Medical Science using microbial culture and colony count in 2019. Alborz University Medical Journal. 2020;9(4): 31-8.
29. Moradnia M, Noorisepehr M, Ghaderi S, et al. Investigation of microbial contamination in surfaces and waterlines of dental units in terms of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Coliforms. Journal of Environmental Health Engineering. 2021;8(2):185–95.
30. Hoseini mehrian Z, Naghmachi M, Zirakfard F, et al. A study on microbial quality of water used in the dentistry units. Armaghane-danesh. 2014;19(8):707-16.
31. American Dental Association. ADA statement on dental unit waterlines. The Journal of the American Dental Association. 1996;127(2):185-6.
32. Santiago JI, Huntington M, Johnston A, et al. Microbial contamination of dental unit waterlines: short-and long-term effects of flushing. Gen Dent. 1994;42(6):528-35.
33. Khorakian F, Movahhed T, Karbasi S, et al. Frequency evaluation of Pseudomonas SPP contamination in the dental unit water line system at Mashhad dental school. Res Dent Sci. 2019;16(3):209-1.
34. Smith A. The devil is in the validation and design; managing the risk from opportunistic pathogens in the dental unit. J Hosp Infect. 2021;114:61-2.
35. Pankhurst CL, Johnson N, Woods R. Microbial contamination of dental unit waterlines: the scientific argument. Int Dent J. 1998;48(4):359-68.
36. Berlutti F, Testarelli L, Vaia F, et al. Efficacy of anti-retraction devices in preventing bacterial contamination of dental unit water lines. Journal of Dentistry. 2003;31(2):105-10.
Type of Study: Original articles |
Subject:
Environmental Health, Sciences, and Engineering
Received: 2025/05/13 | Accepted: 2025/07/20 | Published: 2025/09/30
Received: 2025/05/13 | Accepted: 2025/07/20 | Published: 2025/09/30
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







