Volume 4, Issue 3 (September 2019)
J Environ Health Sustain Dev 2019, 4(3): 819-833 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Javaheri M, Mokhtati M, Samaei M R, Sedighi Khavidak S, Shamsi F, Zamani P et al . Evaluation the Capability of Isolated Bacteria from Stabilized Compost for Bioremediation of Pyrene and Phenanthrene from Contaminated Soil with Municipal Solid Waste Leachate. J Environ Health Sustain Dev 2019; 4 (3) :819-833
URL: http://jehsd.ssu.ac.ir/article-1-196-en.html
URL: http://jehsd.ssu.ac.ir/article-1-196-en.html
Masoume Javaheri 

 , Mehdi Mokhtati
, Mehdi Mokhtati 

 , Mohammad Reza Samaei
, Mohammad Reza Samaei 

 , Samane Sedighi Khavidak
, Samane Sedighi Khavidak 

 , Farimah Shamsi
, Farimah Shamsi 

 , Parvin Zamani
, Parvin Zamani 

 , Ali Asghar Ebrahimi *
, Ali Asghar Ebrahimi * 




 , Mehdi Mokhtati
, Mehdi Mokhtati 

 , Mohammad Reza Samaei
, Mohammad Reza Samaei 

 , Samane Sedighi Khavidak
, Samane Sedighi Khavidak 

 , Farimah Shamsi
, Farimah Shamsi 

 , Parvin Zamani
, Parvin Zamani 

 , Ali Asghar Ebrahimi *
, Ali Asghar Ebrahimi * 


Environmental Science and Technologhy Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 703 kb]
(682 Downloads)
| Abstract (HTML) (2604 Views)
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are commonly found pollutants in soil 1. The main source of PAHs is human activities, such as incomplete combustion of fossil fuels, oil leakage, refineries, and industries. They are also produced as a result of natural disasters, including forest and pasture fires and volcanoes 1-3.
Since the 1990s, extensive studies have been conducted that show the presence of organic chemical groups, such as phenolic compounds, aromatic acids, chlorinated aromatic compounds, and polycyclic aromatic compounds in municipal landfill leachate.
These materials have drawn attention for their unique properties such as stability; bioaccumulation and toxic effects 4. Most polycyclic compounds are known as toxic, mutagenic and carcinogenic pollutants. The US Environmental Protection Agency (EPA) has classified polycyclic aromatic compounds as priority pollutants 5, 6. In order to have a healthy and sustainable environment, one of the main steps is to eliminate and reduce pollutants in contaminated soil 7. PAHs can be removed from water and soil by various physical, thermal, chemical and biological methods. Among these methods, biological processes have drawn comparably more attention with respect to decomposition of hazardous compounds because they are environmentally friendly and economical 8, 9. Ma et al. managed to isolate a species of Pseudomonas that could degrade and remove fluorine and phenanthrene at 4-37 °C. The findings showed that this strain had a high efficiency in removing fluorine and phenanthrene from the soil of cold regions or in cold seasons 10. Maiti et al. also isolated and identified a species of Bacillus from the soil of a refinery in India. This strain could mineralize anthracene, fluoranthene, pyrene and benzo[a]pyrene and produce biosurfactants 11. In one study done by Eskandari et al. bacteria that were capable of growing and reproducing in the presence of PAHs were identified using biochemical tests and genome sequencing technique. These bacteria belonged to Bacillus leucniformis ATHE9, Bacillus mojavensis ATHE13, and a specific species of Bacillus called ATHE10 12.
The aim of this study was to investigate the removal of PAHs from soil contaminated with municipal solid waste leachate by bacteria isolated from stabilized compost.
Materials and Methods
Soil sampling and measurement of physicochemical properties of soil
Sampling was done in the landfill of Yazd at a depth of 0-15 cm 13. The soil was first passed through a 2 mm sieve to be cleaned and to remove oil substances 1.
Then the soil was washed with distilled water and after drying at 60 °C and reaching constant weight, was sterilized at 1.1 bar pressure for 60 min, and then washed with normal hexane several times. The soil was left at 60 °C to completely evaporate hexane 8.
Before the soil was washed, the saturated extract was prepared from it, and the moisture content, organic matter, electrical conductivity and acidity of the soil as well as the amounts (%) of nitrogen, phosphorus, and potassium, clay, sand and silt in soil sample measured 14,15.
Soil contamination
After preparation of the soil sample, to achieve the uniform distribution of pyrene and phenanthrene in it, the required amount of pyrene was dissolved in 80 ml of hexane given the concentrations to be studied and added to the soil sample.
To achieve homogeneous absorption, the mixture was well stirred, and for solvent evaporation, placed under the hood and stirred several times during drying to further ensure homogeneity. Then, the soil was allowed one week to completely absorb pyrene and phenanthrene 8. Concentrations of 10, 25, 40 and 55 µg/kg of soil contaminated with PAHs were used.
Isolation, purification and identification of bacteria in stabilized compost
Isolation of bacteria from stabilized compost, as well as purification and identification of bacteria were done according to the procedure of Samaei et al. Identification was performed using the 16S rDNA sequencing method. First, DNA extraction was done using the kit.
For the 16SrDNA gene amplification,
the forward primers 16F27:5, AGATTTG ATCMTGGCTCAG-3 and 16R1488: 5,-CGGTTACCTTGTTAGGACTTCACC-3 were used. The PCR was performed on a thermocycler (MJ Mini). PCR products were electrophoresed. DNA fragments were observed and photographed by means of the Uvidoc device. Finally, PCR products were sent to the Gene Faravaran Company for sequencing. Then using the BLAST software, the closest sequences to the bacteria were determined, and their genus and species identified 9.
Preparation of mineral culture medium
A mineral culture medium containing elements (g/L) below was prepared: Dipotassium phosphate 0.8, monopotassium phosphate 0.2, calcium sulfate dihydrate 0.05, magnesium sulfate 1.02, iron sulfate 7 hydrate 0.09, ammonium sulfate 1 and small elements manganese chloride tetrahydrate 0.04, molybdenum trioxide 0.08, copper sulfate 0.006, zinc sulfate 0.1, and boric acid 0.00003 9. All of the used materials were taken of Merck Co. (Germany).
Investigating the ability of bacteria and selecting the most potent bacteria
The exact number of bacteria was determined at an optical density equal to one (OD = 1) and 595-nm wavelength. One ml of the medium was poured into the erlenmeyer flasks containing 40 mL of liquid mineral culture medium and 10 g of contaminated soil at 100 mg/kg added (slurry mode). The erlenmeyer flasks were shaken at 150 rpm in a shaker incubator at 30°C. This step was performed in duplicate. The control sample contained no bacteria and 0.2 g of sodium azide was added to it. After 5 and 10 days, the amounts of remaining PAHs were measured using the GC-MS instrument. In addition, the solutions in the Erlenmeyer flask on the shaker incubator were cultured on the nutrient agar every five days, and the pH was also measured. Plates were then incubated at 30 °C for 24 hours. The number of colonies was determined using colony counter. The bacterial culture was also prepared at dilutions 10-2 and 10-3 8, 9, 16. The selected bacteria were exposed to 10, 25, 40, 55 μg/kg of soil contaminated separately with pyrene and phenanthrene. This period lasted 30 days. The amounts of pyrene and phenanthrene were measured on days 0, 15 and 30.
The microbial consortium's ability to remove pyrene and phenanthrene
The ability of the microbial consortium to biodegrade 55 µg/kg of pyrene and phenanthrene in the soil was investigated. The exact number of bacteria was determined at OD = 1 and 595 nm wavelength. The only difference was that the inoculation of microbial suspension into the culture medium was conducted using a mixture of bacterial strains. Erlenmeyer flask containing no bacteria and 0.2 g sodium azide was considered as control 17, 18.
Study of the growth kinetics of the most potent bacteria
For this purpose, two major bacterial species and their consortium were examined. Phenanthrene and pyrene were prepared at concentrations of 100 mg/L. The exact number of more potent bacteria in removing each of the compounds at OD = 1 and 595-nm wavelength was added to the erlenmeyer flasks containing the mineral culture medium and PAHs. The erlenmeyer flasks were left at 30 °C and shaken at 150 rpm for 10 days. Using optical spectroscopy (DR5000) at 595-nm wavelength, the OD was measured every 24 hours 19.
Measuring the amounts of pyrene and phenanthrene
The PAHs were extracted using dichloromethane solvent. The remaining amounts of pyrene and phenanthrene were measured using the GC-MS instrument (7890A Agilent, USA), equipped with MSD detector (C 5975 Agilent, USA), and device (DB-5MS) column characteristics were as follows: 60 meters long, 0.25 mm in diameter and 0.25 microns thick. The detector was set at the SIM mode and 178 m/z was selected for pyrene and 22 m/z for phenanthrene.
The ionization was carried out in EI mode at 70 ev, and the operating conditions of the device were as follows: the injector was set at splitless 1.10, 280 °C, the sample injection rate was 2 μl, and the oven temperature was applied such that the initial temperature was 120 °C with a gradient of 20 °C min-1 to the final temperature of 300 °C and a 4 minutes stop at this temperature. Helium was used as carrier gas at a flow rate of 2 mL/min.
Statistical analysis
One-way ANOVA was used to determine the removal efficiency pyrene by bacteria. The SPSS software version 24 and Mann-Whitney test were also used to evaluate the mean values for single bacteria and consortium.
Ethical issues
Ethical approval was obtained from the Ethics Committee of Shahid Sadoughi University of Medical sciences, Yazd, Iran (ID: IR.SSU. SPH.REC.1396.76).
Results
Soil physicochemical characteristics
The physicochemical characteristics of
soil sample were investigated in this study
(Table 1).
.png)
Figure 1: The degradation efficiency of pyrene by five bacterial strains isolated from stabilized compost
.png)
Figure 2: The degradation efficiency of phenanthrene by five bacterial strains isolated from stabilized compost
Table 2: The amounts of remaining pyrene on days 5 and 10 (initial concentration: 100 mg/kg) after the beginning of degradation
Table 3: The amounts of remaining phenanthrene on days 5 and 10 (initial concentration: 100 mg/kg)
after the beginning of degradation
.png)
Figure 4: Logarithmic growth of single strains and bacterial consortium exposed to phenanthrene (at 55 μg/kg)
.png)
Figure 5: Logarithmic growth of Paenibacillus exposed to different concentrations of pyrene (g/kg) over 30 days
.png)
Figure 6: Logarithmic growth of single strains and bacterial consortium exposed to pyrene (at 55 μg/kg)
.png)
Figure 7: The biodegradation efficiency of pyrene by Paenibacillus at different concentrations (μg/kg)
Table 4: The amounts of remaining pyrene in erlenmeyer flasks over time
.png)
Figure 8: The removal efficiency of pyrene by single strains and bacterial consortium (at 55 μg/kg)
Table 5: Comparison of pyrene remaining concentrations over time after removal by single strains and bacterial consortium
.png)
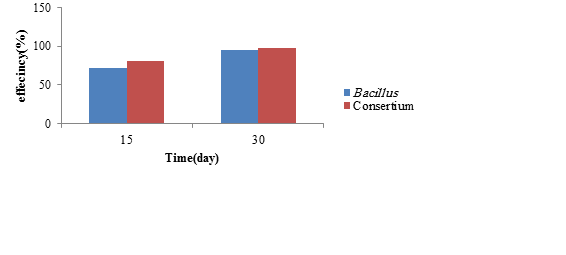
Figure 10: The biodegradation efficiency of phenanthrene by single strains and bacterial consortium (55 μg/kg)
Table 7: The comparison of the concentrations of phenanthrene over time after removal
by single strains and bacterial consortium
.png)
Figure 11: The growth kinetics of Bacillus exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
.png)
Figure 12: The growth kinetics of Paenibacillus exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
.png)
Figure 13: The growth kinetics of microbial consortium exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
Full-Text: (911 Views)
Evaluation the Capability of Isolated Bacteria from Stabilized Compost for Bioremediation of Pyrene and Phenanthrene from Contaminated Soil with Municipal Solid Waste Leachate
Masoume Javaheri 1, 2, Mehdi Mokhtati 1, Mohammad Reza Samaei 3, Samane Sedighi Khavidak 4, Farimah Shamsi 5, Parvin Zamani 1, Ali Asghar Ebrahimi 1*
1 Environmental Science and Technologhy Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran.
3 Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Science, Shiraz, Iran.
4 Department of Biology, Khorramabad Branch, Islamic Azad University, Khorramabad, Iran.
5 Department of Statistics, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Masoume Javaheri 1, 2, Mehdi Mokhtati 1, Mohammad Reza Samaei 3, Samane Sedighi Khavidak 4, Farimah Shamsi 5, Parvin Zamani 1, Ali Asghar Ebrahimi 1*
1 Environmental Science and Technologhy Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran.
3 Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Science, Shiraz, Iran.
4 Department of Biology, Khorramabad Branch, Islamic Azad University, Khorramabad, Iran.
5 Department of Statistics, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
| A R T I C L E I N F O | ABSTRACT | |
| ORIGINAL ARTICLE | Introduction: Pyrene and phenanthrene are polycyclic aromatic hydrocarbons (PAHs) are priority pollutants. The aim of this study was to investigate the ability of isolated bacteria from stabilized compost for biodegradation of pyrene and phenanthrene from soil contaminated with municipal solid waste leachate. Materials and Methods: In this study, phenanthrene and pyrene were selected as priority PAHs pollutants. The degrading bacteria of these compounds were isolated from the stabilized compost and their ability to remove different concentrations of pyrene and phenanthrene investigated. The remaining concentrations of pyrene and phenanthrene were measured by GC-MS. The growth kinetics of bacteria were also studied using a spectrophotometer at 595-nm wavelength. One-way ANOVA was used to determine the statistical significance and plot the related curves, and the Excel software and SPSS version 24 to do statistical analysis. Results: The most potent bacteria in removing pyrene and phenanthrene were Paenibacillus and Bacillus, respectively. The concentrations to be studied were determined as 10, 25, 40 and 55 μg/kg according to the concentrations of PAHs in the soil contaminated with municipal solid waste leachate. At constant temperature of 30 °C after 30 days at these concentrations, the removal efficiency was, respectively, 96.3%, 83%, 77.8%, and 72.4% for pyrene and 100%, 99.6%, 95.9%, and 94.6% for phenanthrene. The growth kinetics of phenanthrene-exposed bacteria were better than those of pyrene-exposed bacteria. Conclusion: The results show that bacteria in stabilized compost are able to remove pyrene and phenanthrene. The bacteria of the highest efficiency for removal of pyrene and phenanthrene are Paenibacillus and Bacillus, respectively. |
|
| Article History: Received: 25 April 2019 Accepted: 10 July 2019 |
||
| *Corresponding Author: Ali Asghar Ebrahimi Email: ebrahimi20007@gmail.com Tel: +983531492273 |
||
| Keywords: Polycyclic Aromatic, Environmetal Pollution, Biodegradation, Pyrene, Phenanthrene. |
Citation: Javaheri M, Mokhtati M, Samaei MR, et al. Evaluation the Capability of Isolated Bacteria from Stabilized Compost for Bioremediation of Pyrene and Phenanthrene from Contaminated Soil with Municipal Solid Waste Leachate. J Environ Health Sustain Dev. 2019; 4(3): 819-33.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are commonly found pollutants in soil 1. The main source of PAHs is human activities, such as incomplete combustion of fossil fuels, oil leakage, refineries, and industries. They are also produced as a result of natural disasters, including forest and pasture fires and volcanoes 1-3.
Since the 1990s, extensive studies have been conducted that show the presence of organic chemical groups, such as phenolic compounds, aromatic acids, chlorinated aromatic compounds, and polycyclic aromatic compounds in municipal landfill leachate.
These materials have drawn attention for their unique properties such as stability; bioaccumulation and toxic effects 4. Most polycyclic compounds are known as toxic, mutagenic and carcinogenic pollutants. The US Environmental Protection Agency (EPA) has classified polycyclic aromatic compounds as priority pollutants 5, 6. In order to have a healthy and sustainable environment, one of the main steps is to eliminate and reduce pollutants in contaminated soil 7. PAHs can be removed from water and soil by various physical, thermal, chemical and biological methods. Among these methods, biological processes have drawn comparably more attention with respect to decomposition of hazardous compounds because they are environmentally friendly and economical 8, 9. Ma et al. managed to isolate a species of Pseudomonas that could degrade and remove fluorine and phenanthrene at 4-37 °C. The findings showed that this strain had a high efficiency in removing fluorine and phenanthrene from the soil of cold regions or in cold seasons 10. Maiti et al. also isolated and identified a species of Bacillus from the soil of a refinery in India. This strain could mineralize anthracene, fluoranthene, pyrene and benzo[a]pyrene and produce biosurfactants 11. In one study done by Eskandari et al. bacteria that were capable of growing and reproducing in the presence of PAHs were identified using biochemical tests and genome sequencing technique. These bacteria belonged to Bacillus leucniformis ATHE9, Bacillus mojavensis ATHE13, and a specific species of Bacillus called ATHE10 12.
The aim of this study was to investigate the removal of PAHs from soil contaminated with municipal solid waste leachate by bacteria isolated from stabilized compost.
Materials and Methods
Soil sampling and measurement of physicochemical properties of soil
Sampling was done in the landfill of Yazd at a depth of 0-15 cm 13. The soil was first passed through a 2 mm sieve to be cleaned and to remove oil substances 1.
Then the soil was washed with distilled water and after drying at 60 °C and reaching constant weight, was sterilized at 1.1 bar pressure for 60 min, and then washed with normal hexane several times. The soil was left at 60 °C to completely evaporate hexane 8.
Before the soil was washed, the saturated extract was prepared from it, and the moisture content, organic matter, electrical conductivity and acidity of the soil as well as the amounts (%) of nitrogen, phosphorus, and potassium, clay, sand and silt in soil sample measured 14,15.
Soil contamination
After preparation of the soil sample, to achieve the uniform distribution of pyrene and phenanthrene in it, the required amount of pyrene was dissolved in 80 ml of hexane given the concentrations to be studied and added to the soil sample.
To achieve homogeneous absorption, the mixture was well stirred, and for solvent evaporation, placed under the hood and stirred several times during drying to further ensure homogeneity. Then, the soil was allowed one week to completely absorb pyrene and phenanthrene 8. Concentrations of 10, 25, 40 and 55 µg/kg of soil contaminated with PAHs were used.
Isolation, purification and identification of bacteria in stabilized compost
Isolation of bacteria from stabilized compost, as well as purification and identification of bacteria were done according to the procedure of Samaei et al. Identification was performed using the 16S rDNA sequencing method. First, DNA extraction was done using the kit.
For the 16SrDNA gene amplification,
the forward primers 16F27:5, AGATTTG ATCMTGGCTCAG-3 and 16R1488: 5,-CGGTTACCTTGTTAGGACTTCACC-3 were used. The PCR was performed on a thermocycler (MJ Mini). PCR products were electrophoresed. DNA fragments were observed and photographed by means of the Uvidoc device. Finally, PCR products were sent to the Gene Faravaran Company for sequencing. Then using the BLAST software, the closest sequences to the bacteria were determined, and their genus and species identified 9.
Preparation of mineral culture medium
A mineral culture medium containing elements (g/L) below was prepared: Dipotassium phosphate 0.8, monopotassium phosphate 0.2, calcium sulfate dihydrate 0.05, magnesium sulfate 1.02, iron sulfate 7 hydrate 0.09, ammonium sulfate 1 and small elements manganese chloride tetrahydrate 0.04, molybdenum trioxide 0.08, copper sulfate 0.006, zinc sulfate 0.1, and boric acid 0.00003 9. All of the used materials were taken of Merck Co. (Germany).
Investigating the ability of bacteria and selecting the most potent bacteria
The exact number of bacteria was determined at an optical density equal to one (OD = 1) and 595-nm wavelength. One ml of the medium was poured into the erlenmeyer flasks containing 40 mL of liquid mineral culture medium and 10 g of contaminated soil at 100 mg/kg added (slurry mode). The erlenmeyer flasks were shaken at 150 rpm in a shaker incubator at 30°C. This step was performed in duplicate. The control sample contained no bacteria and 0.2 g of sodium azide was added to it. After 5 and 10 days, the amounts of remaining PAHs were measured using the GC-MS instrument. In addition, the solutions in the Erlenmeyer flask on the shaker incubator were cultured on the nutrient agar every five days, and the pH was also measured. Plates were then incubated at 30 °C for 24 hours. The number of colonies was determined using colony counter. The bacterial culture was also prepared at dilutions 10-2 and 10-3 8, 9, 16. The selected bacteria were exposed to 10, 25, 40, 55 μg/kg of soil contaminated separately with pyrene and phenanthrene. This period lasted 30 days. The amounts of pyrene and phenanthrene were measured on days 0, 15 and 30.
The microbial consortium's ability to remove pyrene and phenanthrene
The ability of the microbial consortium to biodegrade 55 µg/kg of pyrene and phenanthrene in the soil was investigated. The exact number of bacteria was determined at OD = 1 and 595 nm wavelength. The only difference was that the inoculation of microbial suspension into the culture medium was conducted using a mixture of bacterial strains. Erlenmeyer flask containing no bacteria and 0.2 g sodium azide was considered as control 17, 18.
Study of the growth kinetics of the most potent bacteria
For this purpose, two major bacterial species and their consortium were examined. Phenanthrene and pyrene were prepared at concentrations of 100 mg/L. The exact number of more potent bacteria in removing each of the compounds at OD = 1 and 595-nm wavelength was added to the erlenmeyer flasks containing the mineral culture medium and PAHs. The erlenmeyer flasks were left at 30 °C and shaken at 150 rpm for 10 days. Using optical spectroscopy (DR5000) at 595-nm wavelength, the OD was measured every 24 hours 19.
Measuring the amounts of pyrene and phenanthrene
The PAHs were extracted using dichloromethane solvent. The remaining amounts of pyrene and phenanthrene were measured using the GC-MS instrument (7890A Agilent, USA), equipped with MSD detector (C 5975 Agilent, USA), and device (DB-5MS) column characteristics were as follows: 60 meters long, 0.25 mm in diameter and 0.25 microns thick. The detector was set at the SIM mode and 178 m/z was selected for pyrene and 22 m/z for phenanthrene.
The ionization was carried out in EI mode at 70 ev, and the operating conditions of the device were as follows: the injector was set at splitless 1.10, 280 °C, the sample injection rate was 2 μl, and the oven temperature was applied such that the initial temperature was 120 °C with a gradient of 20 °C min-1 to the final temperature of 300 °C and a 4 minutes stop at this temperature. Helium was used as carrier gas at a flow rate of 2 mL/min.
Statistical analysis
One-way ANOVA was used to determine the removal efficiency pyrene by bacteria. The SPSS software version 24 and Mann-Whitney test were also used to evaluate the mean values for single bacteria and consortium.
Ethical issues
Ethical approval was obtained from the Ethics Committee of Shahid Sadoughi University of Medical sciences, Yazd, Iran (ID: IR.SSU. SPH.REC.1396.76).
Results
Soil physicochemical characteristics
The physicochemical characteristics of
soil sample were investigated in this study
(Table 1).
Table 1: Soil physicochemical characteristics
| Results | Characteristics |
| 7.4 | PH |
| 3.05 | Electrical conductance (ms/cm) |
| 1.83 | Organic compounds (%) |
| 0.262 | Moisture (%) |
| 0.465 | Nitrogen (%) |
| 0.309 | Phosphorus (%) |
| 0.43 | Potassium (%) |
| 30 | Sand (%) |
| 65 | Clay (%) |
| 5 | Silt (%) |
| Clay-sand | Soil tissue |
The results regarding more potent strains among the five strains isolated from stabilized compost
The strains studied were Ochrobactrum oryzae, Bacillus, Sphingomonas sp, Paenibacillus latus, and Serratia marcescens. The removal efficiency of pyrene and phenanthrene at 100 mg/kg of soil by these strains are illustrated in Figures 1 and 2. Our results showed that Paenibacillus and Bacillus showed better efficiency in removing pyrene and phenanthrene, respectively, than other strains.
Based on the one-way ANOVA results, there was a significant difference in the average removal efficiency of pyrene by the five bacterial species on day 5 (P < 0.05), but no significant difference in this variable on day 10 (P > 0.05).
According to the one-way ANOVA results, there was also a significant difference in the average removal efficiency of phenanthrene by
the five bacterial species on days 5 and 10
(P < 0.05).
The remaining concentrations of pyrene and phenanthrene on days 5 and 10 are shown in Tables 2 and 3.
The strains studied were Ochrobactrum oryzae, Bacillus, Sphingomonas sp, Paenibacillus latus, and Serratia marcescens. The removal efficiency of pyrene and phenanthrene at 100 mg/kg of soil by these strains are illustrated in Figures 1 and 2. Our results showed that Paenibacillus and Bacillus showed better efficiency in removing pyrene and phenanthrene, respectively, than other strains.
Based on the one-way ANOVA results, there was a significant difference in the average removal efficiency of pyrene by the five bacterial species on day 5 (P < 0.05), but no significant difference in this variable on day 10 (P > 0.05).
According to the one-way ANOVA results, there was also a significant difference in the average removal efficiency of phenanthrene by
the five bacterial species on days 5 and 10
(P < 0.05).
The remaining concentrations of pyrene and phenanthrene on days 5 and 10 are shown in Tables 2 and 3.
.png)
Figure 1: The degradation efficiency of pyrene by five bacterial strains isolated from stabilized compost
.png)
Figure 2: The degradation efficiency of phenanthrene by five bacterial strains isolated from stabilized compost
Table 2: The amounts of remaining pyrene on days 5 and 10 (initial concentration: 100 mg/kg) after the beginning of degradation
| Remaining concentration after day 10 (mg/kg) |
Remaining concentration after day 5 (mg/kg) |
Bacterial strain |
| 30.74 | 36.73 | Ochrobacterum oryzae |
| 31.29 | 37.42 | Bacillus |
| 27.12 | 43.58 | Sphingomonas |
| 23.56 | 24.21 | Paenibacillus lautus |
| 27.39 | 29.56 | Serratia marcescens |
| 96.6 | 98.1 | Control (without bacteria) |
Table 3: The amounts of remaining phenanthrene on days 5 and 10 (initial concentration: 100 mg/kg)
after the beginning of degradation
| Remaining concentration after day 10 (mg/kg) |
Remaining concentration after day 5 (mg/kg) |
Bacterial strain |
| 34.09 | 36.92 | Ochrobacterum oryzae |
| 20.74 | 31.81 | Bacillus |
| 40.71 | 41.75 | Sphingomonas |
| 26.01 | 32.61 | Paenibacillus lautus |
| 27.56 | 35.92 | Serratia marcescens |
| 95.97 | 97.8 | Control (without bacteria) |
pH value in during operation time
In this regard, every 5 days, the pH of the solutions in the erlenmeyer flasks was measured in during operation time, and the pH value was found to be stable through 30 days (constant range of 7 ± 0.5)
The growth of bacteria exposed to soil contaminated with phenanthrene and pyrene in during operation time
The results regarding the 30-day culture of the erlenmeyer flasks on the shaker incubator on the nutrient agar over every 5 days are shown in figures 3, 4, 5 and 6.
Figures 3 and 5 illustrate the effect of different concentrations of phenanthrene and pyrene on the growth of bacteria in during operation period.
As illustrated, the growth of bacteria in the first days was slow but increased over time, then decreased and eventually became constant, with higher growth at lower concentrations.
The bacterial consortium (Paenibacillus and Bacillus) at 55 μg/kg of phenanthrene-contaminated soil had higher growth rate than Bacillus alone (Figure 4), and the microbial consortium (Paenibacillus and S. marcescens) at 55 μg/kg of pyrene-contaminated soil had higher growth rate than Paenibacillus alone (Figure 6).
.png)
In this regard, every 5 days, the pH of the solutions in the erlenmeyer flasks was measured in during operation time, and the pH value was found to be stable through 30 days (constant range of 7 ± 0.5)
The growth of bacteria exposed to soil contaminated with phenanthrene and pyrene in during operation time
The results regarding the 30-day culture of the erlenmeyer flasks on the shaker incubator on the nutrient agar over every 5 days are shown in figures 3, 4, 5 and 6.
Figures 3 and 5 illustrate the effect of different concentrations of phenanthrene and pyrene on the growth of bacteria in during operation period.
As illustrated, the growth of bacteria in the first days was slow but increased over time, then decreased and eventually became constant, with higher growth at lower concentrations.
The bacterial consortium (Paenibacillus and Bacillus) at 55 μg/kg of phenanthrene-contaminated soil had higher growth rate than Bacillus alone (Figure 4), and the microbial consortium (Paenibacillus and S. marcescens) at 55 μg/kg of pyrene-contaminated soil had higher growth rate than Paenibacillus alone (Figure 6).
.png)
Figure 3: Logarithmic growth of Bacillus exposed to different concentrations of phenanthrene over 30 days.
.png)
Figure 4: Logarithmic growth of single strains and bacterial consortium exposed to phenanthrene (at 55 μg/kg)
.png)
Figure 5: Logarithmic growth of Paenibacillus exposed to different concentrations of pyrene (g/kg) over 30 days
.png)
Figure 6: Logarithmic growth of single strains and bacterial consortium exposed to pyrene (at 55 μg/kg)
Removal of pyrene by more potent bacteria and bacterial consortium
At this step, Paenibacillus already found to be the most potent bacterium in removing pyrene was exposed to contaminated soil with different concentrations of this substance. The results on the removal efficiency are illustrated in Figure 7 and the results regarding the remaining concentrations presented in Table 4.
Based on the one-way ANOVA results, the correlation between the removal efficiency of pyrene by Paenibacillus and the variable time was significantly direct and strong (P ≤ 0.001).
In fact, based on this test, the removal efficiency increased over time, and also there was a significant relationship between the removal efficiency of pyrene and different concentrations based on the post hoc test (P < 0.05).
Regarding the consortium of Paenibacillus and S. marcescens that were found as the more potent bacteria in removing pyrene, they were exposed to soil contaminated with pyrene at 55 μg/kg. Figure 8 illustrates the removal efficiency and Table 5 shows the remaining concentrations of pyrene.
As illustrated in Figure 8, the bacterial consortium shows a better efficiency in biodegrading pyrene. According to the Mann-Whitney test results, there was no significant difference in the mean removal efficiency of pyrene by bacteria on days 15 and 30 (P > 0.05).
At this step, Paenibacillus already found to be the most potent bacterium in removing pyrene was exposed to contaminated soil with different concentrations of this substance. The results on the removal efficiency are illustrated in Figure 7 and the results regarding the remaining concentrations presented in Table 4.
Based on the one-way ANOVA results, the correlation between the removal efficiency of pyrene by Paenibacillus and the variable time was significantly direct and strong (P ≤ 0.001).
In fact, based on this test, the removal efficiency increased over time, and also there was a significant relationship between the removal efficiency of pyrene and different concentrations based on the post hoc test (P < 0.05).
Regarding the consortium of Paenibacillus and S. marcescens that were found as the more potent bacteria in removing pyrene, they were exposed to soil contaminated with pyrene at 55 μg/kg. Figure 8 illustrates the removal efficiency and Table 5 shows the remaining concentrations of pyrene.
As illustrated in Figure 8, the bacterial consortium shows a better efficiency in biodegrading pyrene. According to the Mann-Whitney test results, there was no significant difference in the mean removal efficiency of pyrene by bacteria on days 15 and 30 (P > 0.05).
.png)
Figure 7: The biodegradation efficiency of pyrene by Paenibacillus at different concentrations (μg/kg)
Table 4: The amounts of remaining pyrene in erlenmeyer flasks over time
| Concentration Type of bacterium |
Initial concentration μg/kg |
Remaining concentration on day 0 μg/kg | Remaining concentration on day 15 μg/kg | Remaining concentration on day30 μg/kg |
| Paenibacillus lautus | 10 | 9.89 | 3.081 | 0.359 |
| without bacteria | Control | 9.93 | 9.79 | 9.54 |
| Paenibacillus lautus | 25 | 24.81 | 8.62 | 4.21 |
| without bacteria | Control | 24.85 | 24.29 | 23.99 |
| Paenibacillus lautus | 40 | 39.7 | 14.83 | 8.81 |
| without bacteria | Control | 39.79 | 39.01 | 38.32 |
| Paenibacillus lautus | 55 | 54.52 | 22.31 | 15.04 |
| without bacteria | Control | 54.62 | 54.2 | 53.55 |
.png)
Figure 8: The removal efficiency of pyrene by single strains and bacterial consortium (at 55 μg/kg)
Table 5: Comparison of pyrene remaining concentrations over time after removal by single strains and bacterial consortium
| Concentration Type of bacterium |
Initial concentration μg/kg |
Remaining concentration on day 0 μg/kg |
Remaining concentration on day 15 μg/kg |
Remaining concentration on day30 μg/kg |
| Paenibacillus lautus | 55 | 54.52 | 22.31 | 15.04 |
| Paenibacillus lautus and Serratia marcescens | 55 | 54.4 | 10.38 | 2.15 |
| without bacteria | 55 | 54.62 | 54.2 | 53.55 |
Removal of phenanthrene by the most potent bacterium and bacterial consortium
In this step, Bacillus already found to be the most potent bacterium in removing phenanthrene was exposed to contaminated soil with different concentrations of phenanthrene.The removal efficiency of phenanthrene is illustrated in Figure 9 and the results regarding its remaining concentrations presented in Table 6. According to the one-way ANOVA results, the correlation between the removal efficiencof phenanthrene by Bacillus and the variable time was statistically significant and direc(P ≤ 0.001). More clearly, this test showed that over time, the efficiency of removal would increase. According to the post hoc test results, there was a significant relationship between the removal efficiency of different concentrations of phenanthrene (P ≤ 0.001). The removal of phenanthrene at 55 μg/kg of contaminated soil was investigated by a bacterial consortium (Bacillus and Paenibacillus lautus), known as the more potent bacteria in removing it. The removal efficiency of phenanthrene is illustrated in Figure 10 and the results regarding its remaining concentration presented in Table 7. Based on the Mann-Whitney test results, there was no significant difference in the mean removal efficiency of phenanthrene by bacteria on days 15 and 30 (P > 0.05).
.png)
Figure 9: The biodegradation efficiency of phenanthrene by Bacillus at different concentrations (in μg/kg)
Table 6: The remaining concentration of phenanthrene over time
Table 6: The remaining concentration of phenanthrene over time
| Concentration Type of bacterium |
Initial concentration μg/kg |
Remaining concentration after day 0 μg/kg |
Remaining concentration after day 15 μg/kg |
Remaining concentration after day30 μg/kg |
| Bacillus | 10 | 9.8 | 0 | 0 |
| without bacteria | Control | 9.88 | 9.6 | 9.43 |
| Bacillus | 25 | 24.67 | 3.26 | 0.088 |
| without bacteria | Control | 24.71 | 24.01 | 23.32 |
| Bacillus | 40 | 39.4 | 10.11 | 1.61 |
| without bacteria | Control | 39.51 | 39.05 | 38.59 |
| Bacillus | 55 | 54.1 | 15.36 | 2.9 |
| without bacteria | Control | 54.3 | 53.03 | 52.25 |

Figure 10: The biodegradation efficiency of phenanthrene by single strains and bacterial consortium (55 μg/kg)
Table 7: The comparison of the concentrations of phenanthrene over time after removal
by single strains and bacterial consortium
| Concentration Type of bacterium |
Initial concentration μg/kg |
Remaining concentration after day start μg/kg |
Remaining concentration after day 15 μg/kg |
Remaining concentration after day30 μg/kg |
| Bcillus | 55 | 54.1 | 15.36 | 2.9 |
| Paenibacillus lautus and Bcillus | 55 | 52.99 | 1.07 | 1.12 |
| without bacteria | 55 | 54.3 | 53.03 | 52.25 |
The growth kinetics of bacteria and their consortium exposed to pyrene and phenanthrene
Our results showed that over 10 days the growth of Bacillus and Paenibacillus exposed to phenanthrene at 100 mg/L was better than that of these bacteria exposed to pyrene (Figures 11 and 12). In other words, the ability of bacteria to tolerate phenanthrene was higher than their ability to tolerate pyrene.
As illustrated in Figure 11, Bacillus shows the highest OD (0.241) in exposure to phenanthrene after 48 hours and the highest OD (0.196) in exposure to pyrene after 24 hours at 595-nm wavelength, and as Figure 12 illustrates, Paenibacillus shows the highest OD (0.247) in exposure to phenanthrene after 48 hours and the highest OD (0.204) in exposure to pyrene after 72 hours at 595-nm wavelength; As illustrated in Figure 13, the bacterial consortium Bacillus and Paenibacillus exposed to phenanthrene shows better growth compared to that exposed to pyrene. At 595 nm wavelength, the OD was 0.254 after 24-hour exposure to phenanthrene, and the consortium showed the highest OD (0.242) after 48 hours at this wavelength.
Our results showed that over 10 days the growth of Bacillus and Paenibacillus exposed to phenanthrene at 100 mg/L was better than that of these bacteria exposed to pyrene (Figures 11 and 12). In other words, the ability of bacteria to tolerate phenanthrene was higher than their ability to tolerate pyrene.
As illustrated in Figure 11, Bacillus shows the highest OD (0.241) in exposure to phenanthrene after 48 hours and the highest OD (0.196) in exposure to pyrene after 24 hours at 595-nm wavelength, and as Figure 12 illustrates, Paenibacillus shows the highest OD (0.247) in exposure to phenanthrene after 48 hours and the highest OD (0.204) in exposure to pyrene after 72 hours at 595-nm wavelength; As illustrated in Figure 13, the bacterial consortium Bacillus and Paenibacillus exposed to phenanthrene shows better growth compared to that exposed to pyrene. At 595 nm wavelength, the OD was 0.254 after 24-hour exposure to phenanthrene, and the consortium showed the highest OD (0.242) after 48 hours at this wavelength.
.png)
Figure 11: The growth kinetics of Bacillus exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
.png)
Figure 12: The growth kinetics of Paenibacillus exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
.png)
Figure 13: The growth kinetics of microbial consortium exposed to phenanthrene and pyrene (100 mg/L)
over 10 days at 595-nm wavelength
Discussion
In this study, the biodegradation of polycyclic aromatic compounds was investigated. To this end, the ability of five bacteria isolated from stabilized compost in removing phenanthrene and pyrene was investigated. Among the isolated bacteria, two bacteria that were comparatively more potent in removing different concentrations of pyrene and phenanthrene were selected.
Because researchers believe that biodegradation is possible only in the presence of a mixture of efficient species and the biodegradation potential of single species is limited 20, then in this study the degradation of phenanthrene and pyrene by mixed bacteria (bacterial consortium) was investigated.
Effect of pH and temperature on the biodegradation process
In this study, according to the continuous measurements, the pH value was observed to be neutral.
One of the factors influencing the biological processes is the pH of the environment. According to studies and operating conditions in many biological processes, it can be concluded that the best pH value in the environment is neutral (7-8), because bacteria and fungi grow in the environments with pH value near neutral. According to studies, pH increase from neutral level leads to significant decline in degradation of soil hydrocarbons, and in some cases acid pH also halts the biological degradation 21.
In the study of Meshram et al. Bacillus cereus isolated from the contaminated soil could degrade tricyclic PAHs at pH of 6.5-7.5, with high degradation efficiency obtained at pH 7.5, and the temperatures studied were 25, 30, and 35 °C 22.
In the present study, the pH of the sample was obtained (7 ± 0.5) during the incubation period.
All experiments were carried out at 30 °C. According to the studies of Kafilzade et al. and Shafiee et al., the optimal conditions for biodegradation are temperature of 30 °C and pH of 7 18, 19.
Selection of more potent strains among strains isolated from stabilized compost
The bacterial strains isolated from the compost, as presented in the results section, consisted of 5 strains, two of which, ie, gram-positive Bacillus strains, were comparatively more potent in removing phenanthrene and pyrene.
According to the available evidence, gram-positive bacteria, particularly Bacillus, serve special functions in biodegradation processes because they have stronger cell envelope
and exhibit greater resistance to the high concentrations of hydrocarbons than gram-negative bacteria 23.
Kafilzade et al. also showed that most of the bacteria degrading tricyclic PAHs, including acenaphthene, were from the Bacillus strain 24.
The growth of bacteria exposed to soil contaminated with phenanthrene and pyrene in during operation time.
According to the present study (Figures 3 and 5), the growth of bacteria at various concentrations was slow at the beginning of the experiment, then increased exponentially, and finally decreased and became stagnant.
Environmental conditions such as shaking rotation are effective in this stage. As illustrated in the Figure, the trend was initially rising and then, the average removal efficiency decreased at 720-hour interval due to the availability of carbon source resulting from exposure to pollutants.
Since the carbon source needed in the environment was provided by these pollutants, the trend had a negative impact on the population, and it is obvious that their populations decreased over time. In the studies of Kumar et al., Janbandhu et al., and Arulazhagan et al., dilution and colony counting techniques were used to study the growth process of bacteria in PAHs 25.
The results of these studies showed that the rate of growth was slow at the beginning of incubation and the bacteria were in a delayed phase.
Exponential growth occurs as a result of the maximum concentration of aromatic hydrocarbons dissolved in an aqueous medium. And when the consumption of PAHs by bacteria exceeds their dissolution rate, exponential growth stops and the bacteria enters the Stationary phase 25.
Phenanthrene and pyrene biodegradation by more potent strains
In the present study, degradation of pyrene and phenanthrene by more potent bacteria showed that over time the removal efficiency increased and at lower concentrations degradation was accelerated and increased.
Furthermore, the degradation efficiency of phenanthrene was greater than that of pyrene. According to the studies of Kafilzade et al. and Shafiee et al. the percentage of phenanthrene degradation is greater than that of pyrene degradation at similar intervals, but with the increase of the number of benzene rings in PAHs, the degradation rate also decreases 18, 19 .
Over time, the efficiency of the degradation of phenanthrene and pyrene increased. This could be due to the ability of the bacteria to decompose the oil compound depending on the bacterial genus and species, as well as incubation duration.
In the study of Meshram et al., this duration was considered to be 10 days. In some studies, the incubation time was 6-10 days, with a degradation efficiency of 55-65% 22.
In addition, in the present study, the removal efficiency of pyrene and phenanthrene decreased with increasing concentration. This could be due to the reduction of bacterial populations as a result of toxicity and reduced growth of the bacterial population 26.
Furthermore, Nourieh et al. in their study observed that the concentration of phenanthrene decreased over time and phenanthrene was removed faster at lower concentrations 1.
However, in the study of Wong et al. the removal rate of phenanthrene was reported to be higher in slurry bioreactors for low concentrations than for high concentrations 27.
Degradation of phenanthrene and pyrene by the microbial consortium in during operation time
According to the results obtained in this study, the microbial consortium was more potent in removing pyrene and phenanthrene than single-strain bacteria and the degradation efficiency of the microbial consortium was higher.
Fazilah et al. showed that the percentage of removal of phenanthrene by microbial consortium was higher than that by single bacterium.
This is because of the fact that PAHs degradation does not often depend much on the metabolism of a specific bacterium but mainly on the ability of the bacterial consortium 28.
Leblond et al. observed that degradation by a single bacterium was difficult due to production of intermediate compounds, but in exposure to the bacterial consortium, intermediate compounds were produced throughout degradation that were then degraded by other bacteria 29.
In addition, the study of Zafra et al. showed that the biodegradation efficiency of the bacterial consortium increased over time 30.
The growth kinetics of bacteria exposed to pyrene and phenanthrene.
The growth kinetics of bacteria exposed to phenanthrene were better than those of bacteria exposed to pyrene. This can be attributed to the tetracyclic structure of pyrene and the tricyclic structure of phenanthrene.
As previously mentioned, the degradation of the tricyclic structure is easier than that of the tetracyclic structure.
Another reason for pyrene stability is it’s
high molecular weight and low solubility in water. Because pollutant biodegradation is possible in the aqueous phase, the low concentration of the pollutant in the aqueous phase reduces it’s bioavailability and stability in the environment 31.
Conclusion
In this study to investigate five isolated bacterial species isolated from stabilized compost and their ability to grow, two bacterial strains Bacillus and Paenibacillus were found as comparatively more potent strains. Paenibacillus was more potent in removing different concentrations of pyrene and Bacillus more potent in removing phenanthrene.
The bacterial consortium (Paenibacillus lautus and S. marcescens strains) showed better efficiency in removing pyrene at 55 mg/kg and the bacterial consortium (Bacillus and Paenibacillus lautus) showed better efficiency in removing phenanthrene.
Acknowledgements
The authors gratefully thank Shahid Sadoughi University of Medical Sciences, Yazd, as well as the Medical Microbiology Research Center of Ilam University of Medical Sciences.
Funding
This study was funded by Shahid Sadoughi University of Medical Sciences.
Conflict of interest
No conflict of interest has been stated by the authors.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work for commercial use.
References
In this study, the biodegradation of polycyclic aromatic compounds was investigated. To this end, the ability of five bacteria isolated from stabilized compost in removing phenanthrene and pyrene was investigated. Among the isolated bacteria, two bacteria that were comparatively more potent in removing different concentrations of pyrene and phenanthrene were selected.
Because researchers believe that biodegradation is possible only in the presence of a mixture of efficient species and the biodegradation potential of single species is limited 20, then in this study the degradation of phenanthrene and pyrene by mixed bacteria (bacterial consortium) was investigated.
Effect of pH and temperature on the biodegradation process
In this study, according to the continuous measurements, the pH value was observed to be neutral.
One of the factors influencing the biological processes is the pH of the environment. According to studies and operating conditions in many biological processes, it can be concluded that the best pH value in the environment is neutral (7-8), because bacteria and fungi grow in the environments with pH value near neutral. According to studies, pH increase from neutral level leads to significant decline in degradation of soil hydrocarbons, and in some cases acid pH also halts the biological degradation 21.
In the study of Meshram et al. Bacillus cereus isolated from the contaminated soil could degrade tricyclic PAHs at pH of 6.5-7.5, with high degradation efficiency obtained at pH 7.5, and the temperatures studied were 25, 30, and 35 °C 22.
In the present study, the pH of the sample was obtained (7 ± 0.5) during the incubation period.
All experiments were carried out at 30 °C. According to the studies of Kafilzade et al. and Shafiee et al., the optimal conditions for biodegradation are temperature of 30 °C and pH of 7 18, 19.
Selection of more potent strains among strains isolated from stabilized compost
The bacterial strains isolated from the compost, as presented in the results section, consisted of 5 strains, two of which, ie, gram-positive Bacillus strains, were comparatively more potent in removing phenanthrene and pyrene.
According to the available evidence, gram-positive bacteria, particularly Bacillus, serve special functions in biodegradation processes because they have stronger cell envelope
and exhibit greater resistance to the high concentrations of hydrocarbons than gram-negative bacteria 23.
Kafilzade et al. also showed that most of the bacteria degrading tricyclic PAHs, including acenaphthene, were from the Bacillus strain 24.
The growth of bacteria exposed to soil contaminated with phenanthrene and pyrene in during operation time.
According to the present study (Figures 3 and 5), the growth of bacteria at various concentrations was slow at the beginning of the experiment, then increased exponentially, and finally decreased and became stagnant.
Environmental conditions such as shaking rotation are effective in this stage. As illustrated in the Figure, the trend was initially rising and then, the average removal efficiency decreased at 720-hour interval due to the availability of carbon source resulting from exposure to pollutants.
Since the carbon source needed in the environment was provided by these pollutants, the trend had a negative impact on the population, and it is obvious that their populations decreased over time. In the studies of Kumar et al., Janbandhu et al., and Arulazhagan et al., dilution and colony counting techniques were used to study the growth process of bacteria in PAHs 25.
The results of these studies showed that the rate of growth was slow at the beginning of incubation and the bacteria were in a delayed phase.
Exponential growth occurs as a result of the maximum concentration of aromatic hydrocarbons dissolved in an aqueous medium. And when the consumption of PAHs by bacteria exceeds their dissolution rate, exponential growth stops and the bacteria enters the Stationary phase 25.
Phenanthrene and pyrene biodegradation by more potent strains
In the present study, degradation of pyrene and phenanthrene by more potent bacteria showed that over time the removal efficiency increased and at lower concentrations degradation was accelerated and increased.
Furthermore, the degradation efficiency of phenanthrene was greater than that of pyrene. According to the studies of Kafilzade et al. and Shafiee et al. the percentage of phenanthrene degradation is greater than that of pyrene degradation at similar intervals, but with the increase of the number of benzene rings in PAHs, the degradation rate also decreases 18, 19 .
Over time, the efficiency of the degradation of phenanthrene and pyrene increased. This could be due to the ability of the bacteria to decompose the oil compound depending on the bacterial genus and species, as well as incubation duration.
In the study of Meshram et al., this duration was considered to be 10 days. In some studies, the incubation time was 6-10 days, with a degradation efficiency of 55-65% 22.
In addition, in the present study, the removal efficiency of pyrene and phenanthrene decreased with increasing concentration. This could be due to the reduction of bacterial populations as a result of toxicity and reduced growth of the bacterial population 26.
Furthermore, Nourieh et al. in their study observed that the concentration of phenanthrene decreased over time and phenanthrene was removed faster at lower concentrations 1.
However, in the study of Wong et al. the removal rate of phenanthrene was reported to be higher in slurry bioreactors for low concentrations than for high concentrations 27.
Degradation of phenanthrene and pyrene by the microbial consortium in during operation time
According to the results obtained in this study, the microbial consortium was more potent in removing pyrene and phenanthrene than single-strain bacteria and the degradation efficiency of the microbial consortium was higher.
Fazilah et al. showed that the percentage of removal of phenanthrene by microbial consortium was higher than that by single bacterium.
This is because of the fact that PAHs degradation does not often depend much on the metabolism of a specific bacterium but mainly on the ability of the bacterial consortium 28.
Leblond et al. observed that degradation by a single bacterium was difficult due to production of intermediate compounds, but in exposure to the bacterial consortium, intermediate compounds were produced throughout degradation that were then degraded by other bacteria 29.
In addition, the study of Zafra et al. showed that the biodegradation efficiency of the bacterial consortium increased over time 30.
The growth kinetics of bacteria exposed to pyrene and phenanthrene.
The growth kinetics of bacteria exposed to phenanthrene were better than those of bacteria exposed to pyrene. This can be attributed to the tetracyclic structure of pyrene and the tricyclic structure of phenanthrene.
As previously mentioned, the degradation of the tricyclic structure is easier than that of the tetracyclic structure.
Another reason for pyrene stability is it’s
high molecular weight and low solubility in water. Because pollutant biodegradation is possible in the aqueous phase, the low concentration of the pollutant in the aqueous phase reduces it’s bioavailability and stability in the environment 31.
Conclusion
In this study to investigate five isolated bacterial species isolated from stabilized compost and their ability to grow, two bacterial strains Bacillus and Paenibacillus were found as comparatively more potent strains. Paenibacillus was more potent in removing different concentrations of pyrene and Bacillus more potent in removing phenanthrene.
The bacterial consortium (Paenibacillus lautus and S. marcescens strains) showed better efficiency in removing pyrene at 55 mg/kg and the bacterial consortium (Bacillus and Paenibacillus lautus) showed better efficiency in removing phenanthrene.
Acknowledgements
The authors gratefully thank Shahid Sadoughi University of Medical Sciences, Yazd, as well as the Medical Microbiology Research Center of Ilam University of Medical Sciences.
Funding
This study was funded by Shahid Sadoughi University of Medical Sciences.
Conflict of interest
No conflict of interest has been stated by the authors.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work for commercial use.
References
- Nourie N, Nasseri S, Rezaeikalantar R, et al. Isolation and study of biodegradiation potential of phenanthrene degrading bacteria. yafte. 2014;3(6): 53-62.
- Reddy MS, Naresh B, Leela T, et al. Biodegradation of phenanthrene with biosurfactant production by a new strain of brevibacillus sp. Bioresour Technol. 2010; 101(20): 7980-3.
- Kaksonen A, Jussila M, Lindström K, et al. Rhizosphere effect of Galega orientalis in oil-contaminated soil. Soil Biol Biochem. 2006; 38(4):817-27.
- Eggen T, Moeder M, Arukwe A. Municipal landfill leachates: a significant source for new and emerging pollutants. Sci Total Environ. 2010; 408(21):5147-57.
- Saponaro S, Bonomo L, Petruzzelli G,et al. Polycyclic aromatic hydrocarbons (PAHs) slurry phase bioremediation of a manufacturing gas plant (MGP) site aged soil. Water Air Soil Pollut. 2002;135(1-4):219-36.
- Amellal N, Portal J-M, Berthelin J. Effect of soil structure on the bioavailability of polycyclic aromatic hydrocarbons with in aggregates of a contaminated soil. Appl Geochem. 2001; 16(14): 1611-9.
- Onifade A, Abubakar F, Ekundayo F. Bioremediation of crude oil polluted soil in the Niger Delta area of Nigeria using enhanced natural attenuation. Res J Appl Sci. 2007; 2(4): 498-504.
- Samaei MR, Mortazavi SB, Bakhshi B, et al. Isolation, genetic identification, and degradation characteristics of n-Hexadecane degrading bacteria from tropical areas in Iran. Appl Geochem. 2013;22(4):1304-12.
- Samaei MR, Mortazavi SB, Bakhshi B, et al.
Isolation and molecular identification of
n-hexadecan degrading bacteria from compost. Geomicrobiol J. 2014;6:320-7. - .Ma J, Xu L, Jia L. Degradation of polycyclic aromatic hydrocarbons by Pseudomonas sp. JM2 isolated from active sewage sludge of chemical plant. Journal of Environmental Sciences. 2012;24(12):2141-8.
- Maiti A, Das S, Bhattacharyya N. High gelatinase activity of a newly isolated polycyclic aromatic hydrocarbon degrading bacteria bacillus weihenstephanensis strain AN1. J Pharm Res. 2013;6(1):199-204.
- Eskandari S, Hoodaji M, Tahmourespour A, et al. Bioremediation potential of indigenous gram-positive bacteria isolated from contaminated soil with polycyclic aromatic hydrocarbons. Journal of Microbial World. 2013;6(1):34-44.
- Mao J, Luo Y, Teng Y, et al. Bioremediation of polycyclic aromatic hydrocarbon-contaminated soil by a bacterial consortium and associated microbial community changes. Int Biodeterior Biodegradation. 2012;70: 141-7.
- Zhang S, Wang Q, Xie S. Microbial community changes in contaminated soils in response to phenanthrene amendment. International Journal of Environmental Science & Technology. 2011; 8(2): 321-30.
- Wu G, Kechavarzi C, Li X, et al. Influence of mature compost amendment on total and bioavailable polycyclic aromatic hydrocarbons in contaminated soils. Chemosphere. 2013;90(8): 2240-6.
- Kumar S, Upadhayay SK, Kumari B, et al. In vitro degradation of fluoranthene by bacteria isolated from petroleum sludge. Bioresour Technol. 2011;102(4):3709-15.
- Koch E. Biodegradation of polycyclic aromatic hydrocarbons by arthrobacter sp. UG50 isolated from petroleum refinery wastes. [dissertation]. University of Guelph, Ontario, Canada. 2011.
- Shafiee P, Shojaosadati SA, Charkhabi
AH. Biodegradation of polycyclic aromatic hydrocarbons by aerobic mixed bacterial culture isolated from hydrocarbon polluted soils. Iranian Journal of Chemistry and Chemical Engineering. 2006;25(3):73-8. - Kafilzadeh F, Hoshyaripour F, Afrough R, et al. Isolation and Identification of Pyrene-degrading Bacteria from Soils around Landfills in Shiraz and Their Growth Kinetic Assay. Journal of Fasa University of Medical Sciences. 2011;1(3):154-9.
- Ringelberg DB, Talley JW, Perkins EJ, et
al. Succession of phenotypic, genotypic, and metabolic community characteristics during in vitro bioslurry treatment of polycyclic aromatic hydrocarbon-contaminated sediments. Appl Environ Microbiol. 2001;67(4): 1542-50. - Alinajafi S, Rahimpour F. TPH bioremediation by microbial consortium isolated from oil contaminated soil. International Congress on Chemical Engineering. 2009.
- Meshram RL, Wate SR. Isolation, characterization and Anthracene mineralization by Bacillus Cereus from petroleum oil depot soil. Ind J Appl Res . 2014;4(5):16-8.
- Ebrahimi M, Fallah A, sarikhani Mr. Isolation and Identification of Oil-Degrading Bacteria from Oil-Polluted Soils and Assessment of Their Growth in the Presence of Gas Oil. Water and Soil Science. 2013;23(1):109-21.
- Kafilzadeh F, Hoseyni SZ, Izedpanah P,et al. Isolation and identification of carcinogen acenaphthene-degrading endemic bacteria from crude oil contaminated soils around Abadan Refinery. Journal of Fasa University of Medical Sciences . 2012;2(3):181-6.
- Janbandhu A, Fulekar M. Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. Journal of Hazardous Materials. 2011; 187(1-3):333-40.
- Yang L, Lai CT, Shieh WK. Biodegradation of dispersed diesel fuel under high salinity conditions. Water Res. 2000;34(13): 3303-14.
- Wong J, Lai K, Wan C, et al. Isolation and optimization of PAH-degradative bacteria from contaminated soil for PAHs bioremediation. Water Air Soil Pollut. 2002;139(1-4):1-13.
- Fazilah A, Darah I, Noraznawati I. Bioremediation of Phenanthrene by Monocultures and Mixed Culture Bacteria Isolated from Contaminated Soil. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering .2016;10(9):512-15
- Leblond JD, Schultz TW, Sayler GS. Observations on the preferential biodegradation of selected components of polyaromatic hydrocarbon mixtures. Chemosphere. 2001;42(4):333-43.
- Zafra G, Taylor TD, Absalón AE, et al. Comparative metagenomic analysis of PAH degradation in soil by a mixed microbial consortium. Journal of hazardous materials. 2016; 318:702-10.
- Jimenez IY, Bartha R. Solvent-augmented mineralization of pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1996;62(7):2311-6.
Type of Study: Original articles |
Subject:
Special
Received: 2019/04/25 | Accepted: 2019/07/10 | Published: 2019/09/23
Received: 2019/04/25 | Accepted: 2019/07/10 | Published: 2019/09/23
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





